1. Background
The number of patients who undergo hemodialysis is rapidly increasing worldwide (1-3) In 2017, 77,892 chronic kidney disease (CKD) patients in Indonesia were reported to be actively on hemodialysis (4). The number almost doubled to 132,142 within a year (5). Along with the rapidly increasing prevalence, hemodialysis patients have higher mortality compared to the general population (6). Compared to the general population, a study demonstrated a 20-time higher mortality rate due to cardiovascular diseases (CVD) in hemodialysis patients, with CVD being the leading cause of mortality in this population (7).
The mechanism of CVD in hemodialysis patients is multifactorial (8). A well-known risk factor is a high level of uric acid (UA) (9, 10). Uric acid is the end product of purine metabolism, which is excreted primarily through kidneys (11). At normal levels, UA acts as an antioxidant; meanwhile, at high levels, UA acts as a pro-oxidant, thus demonstrating a dual role in the development of CVD (12). In hemodialysis patients, UA levels are highly affected by dialysis and changes in dietary intake (12). A preliminary measurement in 13 hemodialysis patients in our hospital showed that the average uric acid level in twice-weekly hemodialysis patients ranged from 8.17 to 9.35 mg/dL. These levels were higher than those reported in a previous study that assessed thrice-weekly hemodialysis and non-hemodialysis subjects (12).
To date, the relationship between UA levels and CVD and the cut-off UA levels capable of causing CVD in hemodialysis patients are still debatable (13). Hyperuricemia is known to be associated with increased oxidative stress, inflammation, and endothelial dysfunction by inhibiting nitric oxide function. A study reported that hyperuricemia increased the risk of all-cause and CVD-related mortality in CKD patients (14). However, despite hyperuricemia being suggested as a risk factor for CVD mortality, it is still unclear whether or not hyperuricemia is an independent risk factor. Thus, the treatment of hyperuricemia in CKD patients is still controversial (15).
In addition to UA, some uremic toxins, including symmetric dimethylarginine (SDMA), were shown to be associated with CVD (16). As a valuable and sensitive marker of renal function, SDMA is also an independent risk factor for CVD and mortality (16), which has been associated with all-cause and CVD-related mortality in hemodialysis patients (16). However, there is still a lack of understanding about the association between UA and SDMA levels in hemodialysis patients.
2. Objectives
This study aimed to assess the relationship between the levels of UA and SDMA, as a marker of CVD, in the subjects undergoing hemodialysis twice weekly.
3. Methods
This cross-sectional study was conducted in the hemodialysis unit of Dr. Cipto Mangunkusumo General Hospital, Jakarta, Indonesia, from January to December 2020. We consecutively included all patients aged 18 years and older undergoing twice-weekly hemodialysis for at least three months in our hospital. The subjects who were already on uric acid lowering therapy, pregnant or lactating women, and patients with a history of malignancy were excluded. History taking, physical examination, and pre-dialysis blood tests were obtained. Body mass index (BMI) was calculated as weight (in kg) divided by the square of height (in m2). Based on the WHO Asia Pacific classification, BMI cut-offs were regarded as the following: normal (18.5 - 22.9 kg/m2), underweight (< 18.5 kg/m2), overweight (23 - 24.9 kg/m2), obese I (25 - 29.9 kg/m2), and obese II (> 30 kg/m2). The patient’s smoking history, the duration of hemodialysis, and the presence of diabetes and hypertension were recorded from medical records. Pre-dialysis blood pressure was measured using Omron HEM-7203 meter and categorized as < 140 mmHg, 140 - 160 mmHg, and > 160 mmHg. Pre-dialysis venous blood samples were collected from each subject and stored in EDTA-containing tubes. The biochemical workup included uric acid, SDMA (liquid chromatography (LC)-Tandem mass spectrometry (MS/MS)), hs-CRP, and fasting plasma glucose (FPG). Based on the mean UA levels obtained from our preliminary observations in 13 patients under twice-weekly hemodialysis, UA level was categorized into > 8 mg/dL and < 8 mg/dL.
Median values with interquartile ranges (IQR) were obtained using the Mann-Whitney U-test or the one-way ANOVA test for bivariate analysis on the continuous variables that were not normally distributed. The correlation between SDMA and UA levels was evaluated using the Pearson test. P-values of < 0.05 were considered statistically significant.
The Ethics Committee of the Faculty of Medicine, Universitas Indonesia, approved the study with the approval number of KET-435/UN2.F1/ETIK/PPM.00.02/2020. All the participants included in this study gave informed consent freely and voluntarily. The participants were given an opportunity to ask their questions, to all of which we provided adequate responses. None of the participants were coerced to give consent.
4. Results
4.1. Characteristics of Subjects
A total of 126 patients under twice-weekly chronic hemodialysis were included in this study. The participants’ median age was 53.6 years old (IQR: 21, min: 17, max: 78), and males constituted 46.03% of the subjects. The median duration of hemodialysis was 48 months (IQR: 71, min: 3, max:360). The major cause of hemodialysis was hypertension (42.05%), diabetes (31.47%), and glomerulonephritis (21.43%). From the Kt/V measurement, only 54.76% of the subjects achieved adequate hemodialysis. We found that the median UA level was 8.4 mg/dL (IQR: 2.6, min: 4.1, max: 13.6), and 72 subjects (57.14%) had UA levels 8 mg/dL or greater. Meanwhile, the median SDMA level in all 126 subjects was 535.5 (312.7) mmol/dL (min: 119.7, max: 1895.5) (Table 1).
| Variables | Total (N = 126) |
|---|---|
| Uric acid, mg/dL | 8.4 (2.6) |
| > 8 | 72 (57.14%) |
| < 8 | 54 (42.86%) |
| SDMA, mmol/dL | 535.5 (312.7) |
| Age, y | 52.5 (21) |
| BMI, kg/m2 | 22.67 (5.27) |
| Male | 58 (46.03%) |
| Smoking | 25 (19.84%) |
| Hemodialysis duration, mo | 48 (71) |
| Diabetes | 37 (29.37%) |
| Hypertension | 112 (88.89%) |
| Glomerulonephritis | 31 (21.43%) |
| Kt/V | 1.86 (0.45) |
| < 1.8 | 57 (45.24%) |
| > 1.8 | 69 (54.76%) |
| hs-CRP, mg/L | 5 (11.65) |
| Pre-dialysis SBP, mmHg | 140 (32) |
| Total cholesterol, mg/dL | 227.5 (77) |
| Fasting plasma glucose, mg/dL | 108 (44) |
aValues are expressed as No. (%) or median (IQR).
4.2. The Association Between UA and SDMA Levels
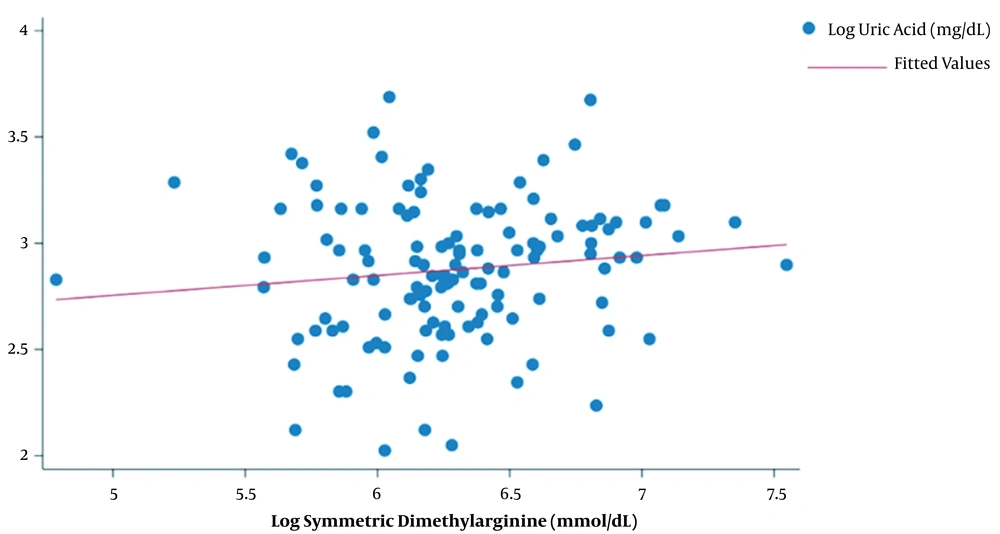
There was no significant correlation between UA and SDMA levels (R = 0.124, P = 0.167) (Figure 1). However, subjects with UA levels > 8 mg/dL had significantly higher SDMA levels compared to subjects with UA levels < 8 mg/dL (550.1 (IQR:357.25) vs 491.35 (IQR:181.1), P = 0.0475). The level of SDMA was also significantly associated with hs-CRP level (P = 0.012). However, age, nutritional status, sex, smoking history, duration of hemodialysis, adequacy of hemodialysis, blood pressure, and blood glucose level were not significantly associated with SDMA levels in hemodialysis patients (Table 2).
| Variables | Total (N = 126), No. (%) | SDMA, mmol/dL, Median (IQR) | P-Value |
|---|---|---|---|
| Uric acid, mg/dL | < 0.05a | ||
| > 8 | 72 (57.14) | 550.1 (357.25) | |
| < 8 | 54 (42.86) | 491.35 (181.1) | |
| Age > 60 years | 41 (32.54) | 526.3 (270.6) | NSa |
| Nutritional status | NSb | ||
| Underweight | 18 (14.29) | 541.95 (274.3) | |
| Normal | 50 (39.68) | 539.1 (286.6) | |
| Overweight | 23 (18.25) | 528.3 (209.2) | |
| Obese I | 26 (20.63) | 457.1 (293.9) | |
| Obese II | 9 (7.14) | 544.2 (551.4) | |
| Male | 58 (46.03) | 563.95 (279.5) | NSa |
| Smoking | 25 (19.84) | 514.2 (196.1) | NSa |
| Hemodialysis duration, mo | NSb | ||
| < 12 | 19 (15.08) | 415.1 (353.3) | |
| 12 - 48 | 45 (35.71) | 515.9 (198.5) | |
| > 48 | 62 (49.21) | 542.9 (306.6) | |
| Kt/V | NSa | ||
| < 1.8 | 58 (46.03) | 515.2 (293.8) | |
| > 1.8 | 68 (53.97) | 535.6 (288.5) | |
| hs-CRP, mg/L | < 0.05b | ||
| < 3.0 | 48 (38.71) | 553.0 (294.5) | |
| 3.0 - 5.0 | 15 (12.10) | 515.9 (307.3) | |
| > 5.0 | 61 (49.19) | 520.4 (293.9) | |
| Pre-dialysis SBP, mmHg | NSb | ||
| < 140 | 57 (45.24) | 550.1 (240.4) | |
| 140 - 160 | 49 (38.89) | 498.1 (262.4) | |
| > 160 | 20 (15.87) | 540.5 (324.5) | |
| Total cholesterol, mg/dL | NSa | ||
| < 200 | 38 (35.19) | 499.3 (253.1) | |
| > 200 | 70 (64.81) | 548.8 (345.4) | |
| Fasting plasma glucose, mg/dL | NSb | ||
| < 80 | 10 (9.35) | 530.5 (289.7) | |
| 80 - 130 | 67 (62.62) | 544.2 (322.6) | |
| > 130 | 30 (28.04) | 530.4 (340.4) |
aMann Whitney U-test.
bOne-way ANOVA.
5. Discussion
This study did not show a significant correlation between UA and SDMA levels in 126 subjects under chronic hemodialysis. However, we found that elevated UA levels (> 8 mg/dL) were associated with higher SDMA levels compared to subjects with UA levels < 8 mg/dL (550.1 (IQR:357.25) vs. 491.35 (IQR:181.1) mmol/dl, P < 0.05).
Our study was the first to evaluate serum SDMA level and its association with serum UA level in subjects under chronic twice-weekly hemodialysis. Previously, only two studies investigated the correlation between UA and SDMA levels. The first study demonstrated that SDMA level positively correlated with UA levels in 58 hyperuricemic adolescents who had normal kidney function (r = 0.34, P < 0.01) (17). Another study found a correlation between SDMA and UA levels in patients with hematological malignancies such as non-Hodgkin's lymphoma (r = 0.59, P = 0.001) and chronic lymphocytic leukemia (r = 0.44, P = 0.041), but not in those suffering from acute myeloid leukemia (18).
Uric acid is a product of purine metabolism and is mainly excreted by kidneys (19). Consequently, hyperuricemia is highly prevalent in CKD patients (20). So, UA serum concentration depends on the rate of purine metabolism and the efficiency of its renal clearance, which is easily affected by dialysis (21).
The clinical implications of hyperuricemia in hemodialysis subjects are still debatable. Studies showed that elevated UA levels were associated with vascular diseases. It was also reported that in the stages III to V of CKD, hyperuricemia is a risk factor for all-cause and CVD-related mortality (22). Uric acids are known for their antioxidant effects, especially in the extracellular environment, and for their pro-oxidant effects in the intracellular environment (23). Increased intracellular urate levels activate protein kinases, NF-KB, growth factors, vasoconstrictors (angiotensin II, thromboxane, and endothelin), and chemokines and induce mitochondrial dysfunction. Uric acid may act as a potent promoter of inflammation at certain levels, and subsequently, inflammation can lead to the generation of yet another uremic toxin (i.e., SDMA).16 However, the link between SDMA and UA levels is still not fully elucidated.
As an uremic toxin, SDMA is a naturally generated amino acid 16 that is removed from the body almost exclusively by kidneys and is more precious than other indicators (such as eGFR) for screening kidney function in certain conditions (16). As a low molecular weight water-soluble uremic toxin (202 Da), SDMA is rapidly cleared during dialysis (16) In our study, the median level of SDMA in the subjects undergoing hemodialysis twice weekly was 535.5 (312.7) mmol/dL (min: 119.7, max: 1895.5). To date, no cut-off levels have been designated for SDMA in hemodialysis patients. The mean level of SDMA in a general population was reported 76.1 (± 21.0) ng/mL, while in patients with uremia, the mean level of SDMA reached 646.4 (± 606.0) ng/mL, 16 which was similar to our findings.
Symmetric dimethylarginine plays a vital role in CKD development and progression. An elevation in SDMA level activates NF-kB and enhances the expression of inflammatory cytokines, including interleukin (IL)-6 and tumor necrosis factor-alpha (TNF-a) (16). It also activates leukocytes by enhancing the generation of reactive oxygen species (ROS) and promotes the creation of modified high-density lipoprotein, causing HDL dysfunctionality (24). However, the prognostic role of SDMA in CKD has not been widely studied. In a meta-analysis on nine studies, increased SDMA levels were not associated with a significant cardiovascular outcome in the general population (RR = 1.32 (95% CI: 0.92 to 1.90) for CVD, RR = 1.44 (95% CI: 0.77 to 2.67) for CHD, and RR = 1.31 (95% CI: 0.83 to 2.07) for stroke) (25).
Recent studies demonstrated the clinical significance of elevated SDMA as an independent risk factor for cardiovascular events in both the general population and CKD patients (26). Symmetric dimethylarginine showed a vital role in the inflammatory process and ROS generation. An in vitro study assessing ten guanidino compounds suggested SDMA as a compound with the most significant role in vascular damage and the secretion of proinflammatory mediators (27). Our study also demonstrated an association between SDMA level and hs-CRP (an inflammatory marker) concentration (p < 0.05).
We did not find a study assessing the relationship between UA and SDMA levels in patients with CKD in the literature. Besides, there is still a lack of information on the SDMA synthesis pathway and its proinflammatory effects. Therefore, in this study, it remains unknown whether or not the elevated level of SDMA in patients with hyperuricemia is a co-existence or a part of a causal relationship.
This study has several limitations. First, due to the nature of the study design, we could not assess the temporal association in our study. Second, we were unable to adjust for confounders that might have attenuated the relationship between UA and SDMA levels, and we did not evaluate CV outcomes. Third, this was a single-center study with a small sample size. Thus, it is still difficult to ascertain if there is a clear linkage between UA and SDMA in hemodialysis patients.
5.1. Conclusions
We found an association between UA and SDMA levels in the subjects undergoing hemodialysis twice weekly, especially those with UA levels of > 8 mg/dL. However, it remains a challenge to determine the role of UA in the metabolic pathway of SDMA. Referring to the study’s limitations, the therapeutic consequences of our findings remain unclear, and other cohort studies are needed to confirm such findings and assess the adverse outcomes of this phenomenon in CKD patients.