1. Background
Diabetes mellitus (DM) imposes a group of common metabolic irregularities that share the phenotype of hyperglycemia (1) associated with microvascular complications (2) including atherosclerosis (3, 4) and coronary artery disease (3). The relationship between metabolic control and development of chronic adverse complications in type 1 diabetes (T1D) has always been a great concern over the years, and nowadays the main indicator of long-term glycemic control in T1D and its complications is glycosylated hemoglobin (HbA1c) (5). Although elevated baseline, mean or updated mean of HbA1c levels have been correlated with diabetic complications (reviewed in (6)), actually several studies have introduced some biomarkers of inflammation and endothelial dysfunction in T1D with or without microvascular complications or diabetic nephropathy (7-9).
YKL-40, also called human cartilage glycoprotein-39 or chitinase 3-like-1(CHI3L1) (10), is a member of mammalian chitinase-like proteins binding to lectin without chitinase activity (11). YKL-40 is secreted by different human cells including inflammatory cells such as neutrophils (12) and macrophages (13). A substantial body of evidence indicates that YKL-40, as an inflammatory marker in different pathologies (14-17), participates in diabetes as well as its complications including endothelial dysfunction (13), atherosclerosis (18), cardiovascular disease (19, 20), and albuminuria (19).
Considering that there are little studies on the correlation of YKL-40 with HbA1C and diabetic nephropathy in T1D (19), we evaluated such correlation to suggest YKL-40 as both diagnostic and prognostic marker of T1D metabolic control as well as of diabetic nephropathy.
2. Methods
Study population: 49 patients with type 1 diabetes not suffering from any diabetic complications during recent 5 years and 43 sex- and age-matched healthy controls participated in the study. The healthy controls with no family history of diabetes were selected from voluntary blood donors of local blood donation organization. The diagnosis of T1D was based on the American diabetes Association’s criteria. Any subject with a history of internal diseases other than diabetes and its complications and use of antihypertensive or lipid-lowering drugs as well as smokes was excluded from the study. The protocol was approved by the local committee of ethics. The study was designed in accordance with the Helsinki declaration. Written informed consent was obtained from all participants older than 16 years or their parents/guardians in the case of younger ones.
Sampling protocol and measurements: Blood and urine samples were taken at 08:00-10:00 in the morning after 12 hours overnight fasting. CBC, serum levels of YKL-40, HbA1c, blood biochemical markers including creatinine, lipid profile, and urinary concentration of albumin and creatinine were measured using standard laboratory methods. Urine albumin and urine/serum creatinine were evaluated through enzymatic immunoassay and Jaffe´ methods, respectively. Albumin/creatinine ratio was quantified in 3 urine samples. A commercial, sandwich type ELISA kit (Quidel, San Diego, CA, USA) was used to measure serum levels of YKL-40 according to kit’s instructions.
Statistical analysis: Variables with normal distribution are presented as mean ± SD. The groups were compared using an unpaired Student’s t test or Welch test. Differences in proportions were tested by the χ2-test or Fisher’s exact test. Pearson’s univariate test was used to correlate between the variables. P < 0.05 was considered statistically significant. All analyses were performed using statistical software package SPSS (version 18; SPSS, Chicago, IL).
3. Results
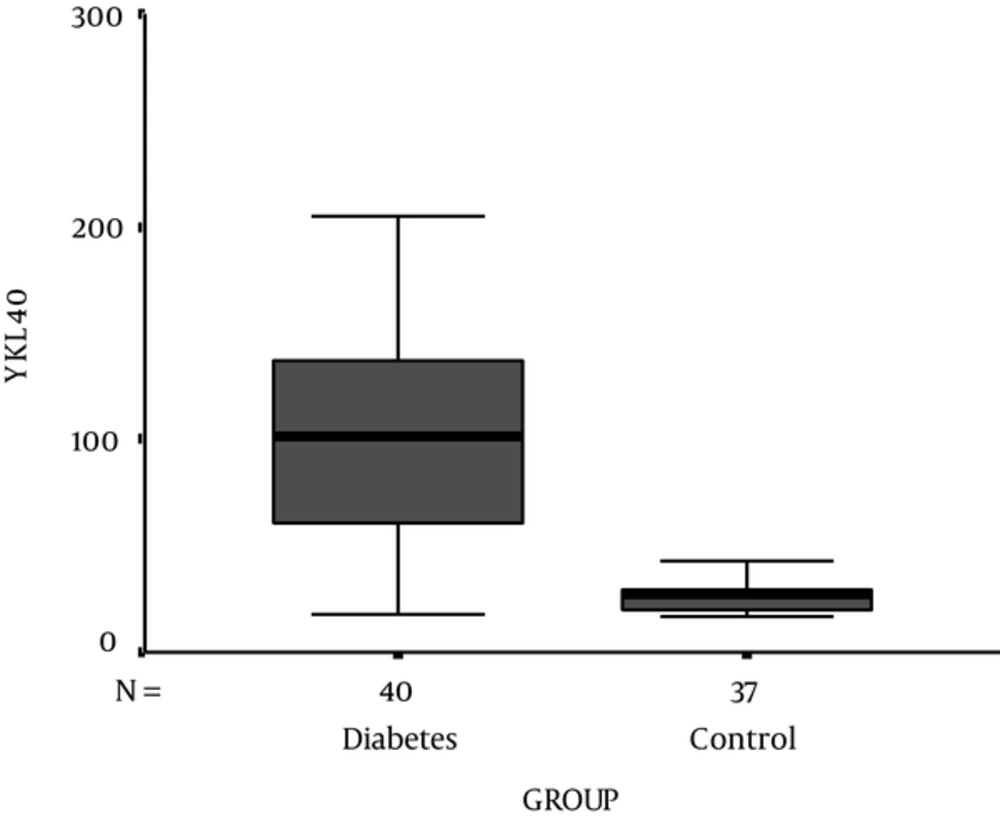
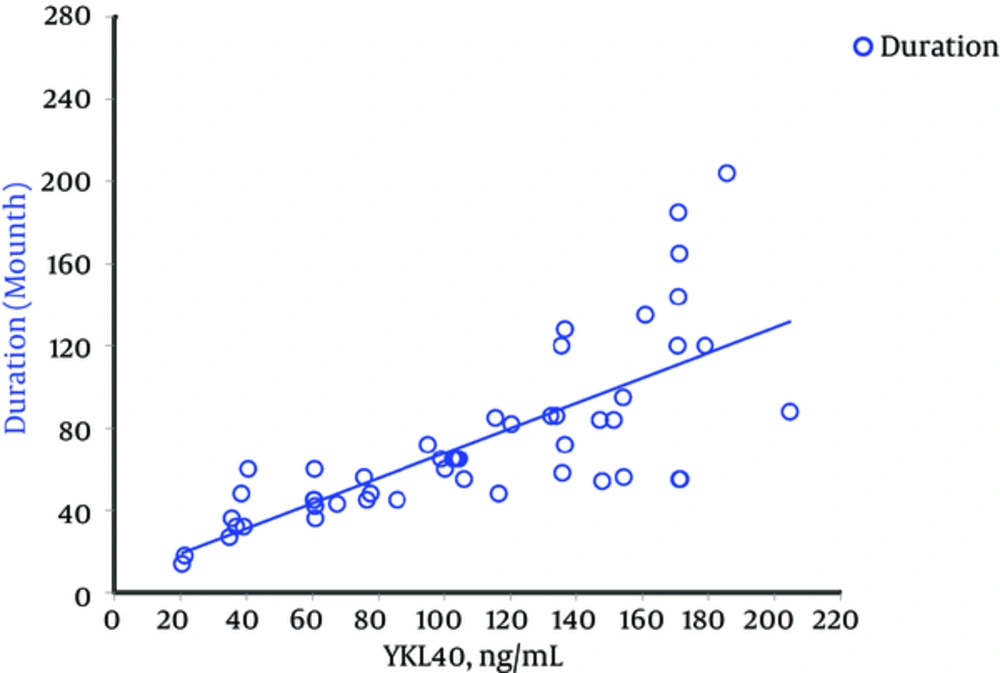
Demographic, experimental, and clinical characteristics of participants are presented in Table 1. HbA1c and serum level of YKL-40 were significantly higher in T1D patients compared to control subjects (P < 0.001) (Figure 1). 83.7% (n = 41) of T1D patients showed a concentration of serum YKL-40 more than the 90% percentile of the healthy controls (42.68 μg/mL). Serum YKL-40 levels were also significantly higher in diabetics according to sex and age compared to those in sex- and age-matched non-diabetics (P < 0.001). There was no significant correlation between the serum levels of YKL-40 and HbA1c (P = 0.99) in T1D patients. Otherwise, it did show strong positive correlations only with duration of the disease (P < 0.001) (Table 2 , Figure 2). In addition, we did not find any significant correlation between YKL-40 serum levels and UACR (as independent marker of diabetic nephropathy), blood pressure, lipid profile, and serum and urine creatinine (Table 2).
| Variants | Healthy Controls (n = 43) | T1D (n = 49) | P Value |
|---|---|---|---|
| Age, y | 10.95 ± 3.83 | 12.20 ± 3.86 | 0.12 |
| Gender (male/female) | 21/22 | 22/27 | 0.7 |
| Duration of diabetes, mo | - | 73.22 ± 5.9 | - |
| BMI, kg/m2 | 21.02 ± 4.25 | 21.23 ± 4.6 | 0.826 |
| Systolic BP, mmHg | 114.33 ± 9.08 | 112.73 ± 10.37 | 0.439 |
| Diastolic BP, mmHg | 75.02 ± 8.96 | 71.53 ± 8.59 | 0.06 |
| HbA1c, % | 4.73 ± 0.73 | 7.34 ± 1.24 | - |
| RBC, Mil/µL | 5 ± 0.52 | 5.18 ± 1.4 | 0.421 |
| Hemoglobin, g/dL | 14.36 ± 1.36 | 13.63 ± 1.16 | 0.007 |
| Hematocrit, % | 43.32 ± 3.62 | 41.18 ± 3.09 | 0.003 |
| Total cholesterol, mg/dL | 159.6 ± 25.2 | 169.3 ± 23.1 | 0.059 |
| Triglyceride, mg/dL | 75.91 ± 27.76 | 77.73 ± 28.4 | 0.758 |
| HDL, mg/dL | 37.67 ± 6.27 | 40.61 ± 7.09 | 0.039 |
| VLDL, mg/dL | 15.86 ± 5.33 | 15.16 ± 5.87 | 0.555 |
| LDL, mg/dL | 106.26 ± 19.6 | 113.24 ± 17.43 | 0.074 |
| Creatinine, mg/dL | 1.07 ± 0.2 | 0.87 ± 0.14 | < 0.001 |
| YKL-40 ng/mL | |||
| Sex | |||
| Male | 28.44 ± 2.37 | 110.54 ± 10.46 | < 0.001 |
| Female | 28.34 ± 1.92 | 107.53 ± 10.11 | < 0.001 |
| P value | 0.97 | 0.84 | - |
| Age, y | |||
| ≤ 12 (n) | 27.03 ± 2.6 (10) | 113.34 ± 9.20 (28) | < 0.001 |
| > 12 (n) | 28.86 ± 1.82 (28) | 102.94 ± 11.67 (21) | < 0.001 |
| P Value | 0.6 | 0.48 | - |
| Total | 28.39 ± 9.84 | 108.88 ± 50.53 | < 0.001 |
| eGFR, mL/min/1.73 m2 | 87.1 ± 34.4 | 90.6 ± 27.8 | 0.64 |
| UACR, mg/g Cr | 3.95 ± 0.43 | 12.16 ± 1.53 | < 0.001 |
| Urine protein, mg/dL | 2.01 ± 2.17 | 1.69 ± 1.52 | 0.538 |
| Urine creatinine, mg/dL | 265.73 ± 158.60 | 136.58 ± 57.97 | < 0.001 |
| Variable | Control Group (P Value) | Diabetic Group (P Value) |
|---|---|---|
| Agea | -0.05 (0.751) | 0.046 (0.752) |
| Duration of diabetesa | - | 0.789 (< 0.001) |
| BMI, kg/m2b | -0.144 (0.359) | 0.282 (0.04) |
| Systolic BP, mmHga | 0.094 (0.548) | -0.279 (0.052) |
| Diastolic BP, mmHga | 0.174 (0.265) | -0.04 (0.783) |
| Hemoglobin, g/dLb | -0.076 (0.627) | -0.018 (0.902) |
| Hematocrit, %b | -0.15 (0.336) | -0.032 (0.829) |
| HbA1c, %b | 0.148 (0.344) | 0.00 (0.995) |
| Total cholesterol, mg/dLb | -0.067 (0.668) | 0.049 (0.738) |
| Triglyceride, mg/dLa | -0.027 (0.866) | 0.182 (0.211) |
| HDL, mg/dLb | 0.012 (0.94) | -0.12 (0.411) |
| VLDL, mg/dLb | -0.1 (0.524) | 0.19 (0.191) |
| LDL, mg/dLb | 0.11 (0.484) | 0.029 (0.841) |
| Creatinine, mg/dLb | -0.366 (0.028) | 0.04 (0.787) |
| Urine Albumin, mg/dLa | -0.029 (0.851) | -0.07 (0.628) |
| Urine creatinine, mg/dLb | 0.008 (0.959) | 0.11 (0.45) |
| UACRa | -0.088 (0.575) | -0.131 (0.368) |
aNon-parametric.
bParametric.
4. Discussion
In line with some other studies (19, 21), we found a higher YKL-40 serum level in the group of T1D patients. Such elevation has also been reported in pre-diabetic (13) as well as in type 2 diabetic patients (13, 20). Moreover, to our knowledge, we report, for the first time, an elevated YKL-40 concentration in a homogenously young population of type 1 diabetics concerning the duration of diabetes with a small standard deviation (73.22 ± 5.9 months) as well as no medical history of diabetic complications and any other diseases.
As a role player of metabolic status (22, 23), it seems that YKL-40 may be a factor influencing HbA1c as a metabolic indicator of diabetes. Having investigated if there was a correlation between HbA1c and serum level of YKL-40, we could not find such correlation. Studying on the correlation of YKL-40 levels with HbA1c in type 2 diabetes, some researchers have found a significant correlation between plasma (24) as well as urine (25) levels of YKL-40 and HbA1c while some others (13, 22, 26, 27) have not found such correlation. In the case of T1D, other studies (20, 21) and ours did not find such correlation.
A significant correlation between YKL-40 plasma levels and age has been a matter of agreement (19, 20, 22, 23, 25, 26, 28-30) or disagreement (14, 31-34) (like ours) in different studies. Such pros (23, 24, 35) (like ours) and cons (13, 14, 19, 27) issues are also true in the case of the significant correlation between YKL-40 plasma levels and BMI.
In agreement (22) and disagreement with some other studies (19, 21), we found no significant correlation between YKL-40 and UACR in T1D patients. This inconsistency may be conceivable considering that our patients were young people suffering for about 6 years from diabetes. Such short duration may be insufficient to develop nephropathic complications.
The advantage of our study was that we used a relatively appropriate sample size and associated standard deviations that made a proper power to detect differences in subgroup analyses. Moreover, there were no essential biases affecting the comparability of the groups including large differences in age, duration of diabetes, and length of treatment. A few studies (19, 21) could show such uniformity of the patients.
We did not monitor the changes of YKL-40 serum levels through serial samples. This would be the main limitation of our cross-sectional study that suffers from the lack of longitudinal data.