1. Background
Hemodialysis requires extracorporeal blood flow. Some form of anticoagulation is used routinely to prevent thrombosis in the blood circuit. Heparin is the anticoagulant of choice for most maintenance hemodialysis worldwide because of its relative ease of use, safety, and low cost (1). Despite all the advantages, the use of long-term heparin is associated with complications including osteoporosis, thrombocytopenia, serum lipid profile changes, platelet dysfunction, and an increased risk of hemorrhage (2, 3).
Low-molecular-weight (LMW) fractions of heparin have recently increased in use and have shown to have a lower incidence of heparin-related adverse events such as thrombocytopenia and osteoporosis. This is due to the reduced binding of LMWH to plasma proteins, platelets, and endothelial cells, which results in fewer complications compared to unfractionated heparin (4, 5). Although LMW heparins do not stimulate plasma lipase activity to the same extent as heparin, their effect on serum lipid remains controversial, as some, but not all, studies report its beneficial effect (6). One of the obstacles to the widespread use of LMW heparin, such as enoxaparin, in hemodialysis is its high cost (7).
The aim of this study was to investigate the safety and clinical efficacy of LMW heparin for hemodialysis anticoagulation in comparison with standard heparin in patients with end-stage renal failure.
2. Methods
This randomized, crossover study with parallel design was conducted in 45 patients who required maintenance hemodialysis due to end-stage renal failure. Hemodialysis was performed via a native arteriovenous fistula thrice weekly for 3 to 5 hours per session at blood flow rates of 250-320 mi/min. Patients (n = 4) with known bleeding disorders, receiving anticoagulant drugs (warfarin, aspirin) and receiving drugs which could affect heparin activity (digitalis, tetracyclines) were excluded. Inclusion criteria consisted of the requirement of hemodialysis and written informed consent. The routine drug regimen prescribed by the physician was continued for all patients during the study. This study was approved by our local ethical committee according to the Helsinki Declaration of the World Medical Association (2000). All patients were informed and were given a written consent form.
Out of 41 patients, 24 were randomly assigned to receive enoxaparin sodium (0.7 mg/kg) and 17 to receive standard heparin for a period of 12 weeks, after which the patients were crossed over to another therapy for a further 12 weeks. The randomization was performed by balanced block randomization with an allocation sequence based on a block size of 8, generated with a computer random number generator. Washout time was 24 hours (5 times the half-life of the drugs studied).
Enoxaparin sodium was administered 5 minutes before dialysis through injection into the pre-dialyser arterial line, whereas heparin was administered with a dose of 50 U/kg intravenously into the pre-dialyser arterial line followed by a maintenance dose of 1000 U per hour. An arterial bubble was visually detected and trapped every 30 minutes.
Dialyser related data (fibrin clot formation) and patient’s clinical data including minor bleeding, vascular compression time, and serum lipid profile were evaluated. Fibrin clot formation, in both the dialyser and arterial lines, was graded on a scale from 1 to 10; with 1 indicating no clot formation and 10 indicating total occlusion. This assessment was carried out after flushing the dialyser and lines with saline in order for the blood to return to the patient. Any bleeding including bleeding at the injection site, epistaxis and bleeding of gingiva was considered as minor bleeding and was observed by an expert nurse. The time between needle removal and cessation of bleeding from the site of puncture was registered as vascular compression time.
A lipid profile including total cholesterol (TC), LDL-cholesterol (LDL), HDL-cholesterol (HDL) and triglycerides (TG) were measured before dialysis in the fasting state and at the end of each arm of the study. Enzyme assay kits (Audit/Ireland), with a sensitivity of 97.8% and a specificity of 94%, were used for diagnosis.
2.1. Statistics
Statistical analysis was performed with SPSS 15 for Windows (SPSS Inc., Chicago, Illinois). Paired data was compared using the paired t-test for parametric and the Mann-Whitney-U test for non-parametric variables. Differences between groups were determine using ANOVA for parametric and Kruskal-Wallis for non-parametric variables. P <0.05 were considered significant.
3. Results
A total of 41 patients who were in end-stage renal failure requiring maintenance hemodialysis were enrolled. Mean age of the study population was of 65.18 (SD = 12.15) years. From these patients, 22 (53%) were male and 19 (47%) were female. The duration of the study was 24 weeks and was divided into 3 phases: 2 different active treatment phases separated by a washout phase.
Patients were randomly assigned to receive either heparin or enoxaparin in a crossover design study. Demographic characteristics of the patients are demonstrated in Table 1; there were no significant differences between the groups.
| Variable | Heparin Group | Enoxaparin Group | P Value |
|---|---|---|---|
| Age | 64.70 ± 10.33 | 65.08 ± 3.59 | 0.2 |
| Sex (M/F) | 13/8 | 9/11 | 0.08 |
| BMI | 25.7 ± 3.50 | 23.1 ± 5.91 | 0.4 |
| Time on dialysis, months | 8.4 ± 2.5 | 7.6 ± 4.1 | 0.9 |
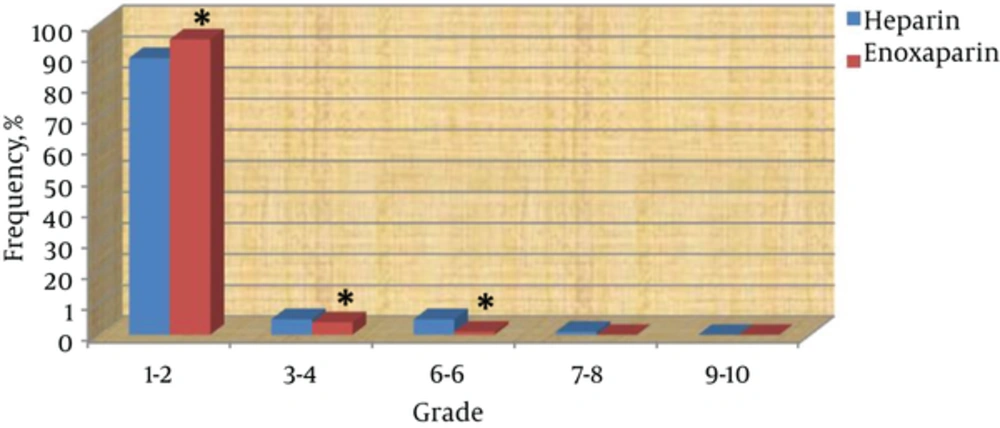
Fibrin clot formation in dialyser and lines is depicted in Figure 1. Of all dialysis sessions, 98% of sessions with enoxaparin and 92% of sessions with heparin were considered to be grade 1 - 2 (P: 0.001). Clot formation in grades 3 - 4 and 5 - 6 were less with enoxaparin than with heparin (P: 0.005 and < 0.0001 respectively). There were no clot formations with grade 8 or higher in both groups.
The frequency of minor bleeding and the mean of vascular compression time at the end of week 12 and week 24 are shown in Table 2. At the end of first study phase, minor bleeding in patients receiving enoxaparin with dose of 0.25 mg/kg was significantly decreased in comparison with patients receiving heparin (P: 0.03), however vascular compression time was not statistically different between the heparin and enoxaparin groups.
| Variable | Heparin Group | Enoxaparin Group | P Value | |
|---|---|---|---|---|
| Week 12 | Minor bleeding, % | 25 | 23 (Dose: 0.7 mg/kg) | 0.07 |
| 17 (Dose: 0.25 mg/kg) | 0.03 | |||
| Vascular compression, min | 2.63 ± 0.22 | 2.60 ± 0.17 | 0.2 | |
| Week 24 | Minor bleeding, % | 14 | 19 (Dose: 0.7 mg/kg) | 0.04 |
| 10 (Dose: 0.25 mg/kg) | 0.01 | |||
| Vascular compression, min | 2.50 ± 0.45 | 2.00 ± 0.50 | 0.1 | |
Abbreviations: HDL, High Density Cholesterol; LDL, Low Density Cholesterol; TC, Total Cholesterol; TG, Triglyceride.
At the end of the second study phase, minor bleeding was significantly increased in the enoxaparin group in comparison with the heparin group (P: 0. 04). In the enoxaparin arm, recurrent blood oozing from puncture sites led to the idea to reduce the dose of enoxaparin. After enoxaparin dose reduction to 0.25 mg/kg, the frequency of minor bleeding decreased to 10% (from 19% to 10%) (P: 0.01). Vascular compression time was not statistically different between the heparin and enoxaparin groups at the end of study (P: 0.1) (Table 2).
There were no significant changes in serum lipids with either anticoagulant, neither at the end of the 12th week nor at the end of the 24th week (Table 3).
| Variable | Heparin | Enoxaparin | P Value | |
|---|---|---|---|---|
| Week 12 | TC (mg/dL) | 142.12 ± 9.17 | 146.10 ± 17.28 | 0.12 |
| TG (mg/dL) | 139.28±48.13 | 137.25 ± 44.47 | 0.63 | |
| HDL (mg/dL) | 36.70 ± 8.423 | 35.18 ± 10.10 | 0.90 | |
| LDL (mg/dL) | 99.56 ± 12.18 | 100.41 ± 28.18 | 0.86 | |
| Week 24 | TC (mg/dL) | 136.25 ± 12.56 | 140.00 ± 21.23 | 0.75 |
| TG (mg/dL) | 136.53 ± 56.5 | 140.12 ± 60.12 | 0.42 | |
| HDL (mg/dL) | 34.18 ± 10.45 | 34.17 ± 7.31 | 0.89 | |
| LDL (mg/dL) | 95.47 ± 21.21 | 101.28 ± 30.11 | 0.65 | |
4. Discussion
The aim of our study was to investigate the use of LMW heparin for hemodialysis anticoagulation in comparison with standard heparin and thereby evaluating the risk of bleeding, clotting formation in the extracorporeal dialysis circuit and their effect on serum lipids.
Our findings show that fibrin clot formation significantly occurred less often in the LMW heparin group than in the standard heparin group, which suggests that in this study enoxaparin was more effective in preventing thrombosis in the dialyser and lines compared to heparin. Abdallah et al. report LMW heparin to be as effective in preventing extracorporeal circuit thrombosis as heparin (5). The meta-analysis by Lim et al. however, contradicts these findings and states that LMWHs do not significantly affect extracorporeal circuit thrombosis when compared to heparin (4).
We found an increased rate of minor bleeding in the LMW heparin group in comparison with heparin notwithstanding they were not severe hemorrhages; this finding only became apparent after several dialyses.
Guillet et al. show that there is an increased risk of bleeding up to 10 hours after administration of enoxaparin (8). Saltissi et al. also report that the early use of enoxaparin can increase minor bleeding in patients who undergo hemodialysis (9).
In our study, after reducing the dose of enoxaparin, minor bleeding decreased significantly in comparison with heparin. Hence we found that a dose of 0.25 mg/kg to be an optimum dose that does not cause clot formation and reduces the frequency of minor bleeding.
Our findings show that the time of vascular compression was not significantly different in heparin and enoxaparin.
Lim et al. show that the time required for arterio-venous fistula compression is not significantly affected by LMW heparin in comparison to heparin (4).
In the current study, there were no significant differences in serum total cholesterol, LDL, HDL and TG after using either heparin or enoxaparin. It seems that because heparin releases lipoprotein lipase from its active site at the capillary endothelial surface (10), it could reduce serum cholesterol, however studies have not yet confirmed this (9-11).
4.1. Conclusion
This study suggests that a single-dose of enoxaparin is an effective and convenient alternative to standard heparin. The recommended dose of this study is 0.25 mg/kg in Iranian patients.