1. Background
Infertility means failure to conception after one year of intercourse. In addition, if a woman is unable to hold the baby in her uterine and an abortion occurs, she is considered infertile. Sometimes the term of sterility is used instead of infertility in women, which affects approximately 10% to 15% of couples in the reproductive age (1). Human infertility factors include bacterial infections (25%), hormonal, genetic, and environmental factors (50%), and unknown causes (25%) (2). Infectious agents can affect the performance and abilities of the reproductive system by direct involvement of different parts of urogenital system, preventing the fertility or successful continue of pregnancy, or reducing fertility rate through systemic effects. The risk of infertility after infection directly depends on the patient's age, number of previous episodes of infection, disease severity at the beginning treatment, and the interval between the start of symptoms to initiation of treatment; the risk of infertility will increase with repeated infection (3). The importance and role of Mycoplasma in female urogenital infections and their complications have been demonstrated (4). Bacteria such as Mycoplasma hominis and Ureaplasma urealyticum are very common bacterial causes of urogenital tracts infections in males and females (5). They decrease the sperm count mobility and increase the percentage of abnormal sperms; moreover, they can cause abortion (6). Studies have shown that PCR is a sensitive, specific, easy, and fast method for detection of M. hominis in genital samples (7, 8). In addition, the relatively high proportion of infertile males has been infected with M. hominis and U. urealyticum (9, 10).
2. Objectives
The aim of this study was to isolate and identify the molecular identity of M. hominis in samples from urogenital tract of infertile males and females referred to the Infertility Center of Kerman, Iran, in 2013.
3. Materials and Methods
In this descriptive study, 200 samples were purposefully collected from males and females who attended the Infertility Center of Kerman during the first six months of 2013. According to WHO standards, 100 semen samples with abnormal spermiogram parameters were collected from infertile males and were kept at -20℃ until DNA extraction. In addition, 100 vaginal swab samples were collected from infertile females by midwives, were completely solved in a PBS solution, and were kept at -20℃ until DNA extraction. A volume of 100 mL of sample was poured into a microtube and then centrifuged for ten minutes at 13000 rpm. The supernatant was discarded and the precipitate was maintained for DNA extraction at the bottom of the microtube. For bacterial DNA extraction, the purification kit, Sinagen Company (CAT NO: PR881613CinnaPure-DNA (Cell culture, Tissues, Gram negative Bacteria, and CSF), 50 Preps were used.
The samples' DNA was extracted according to the Kit manufacturer guidelines and finally, the solution below the column containing the purified DNA was kept at -20℃ until performing PCR.
3.1. Polymerase Chain Reaction
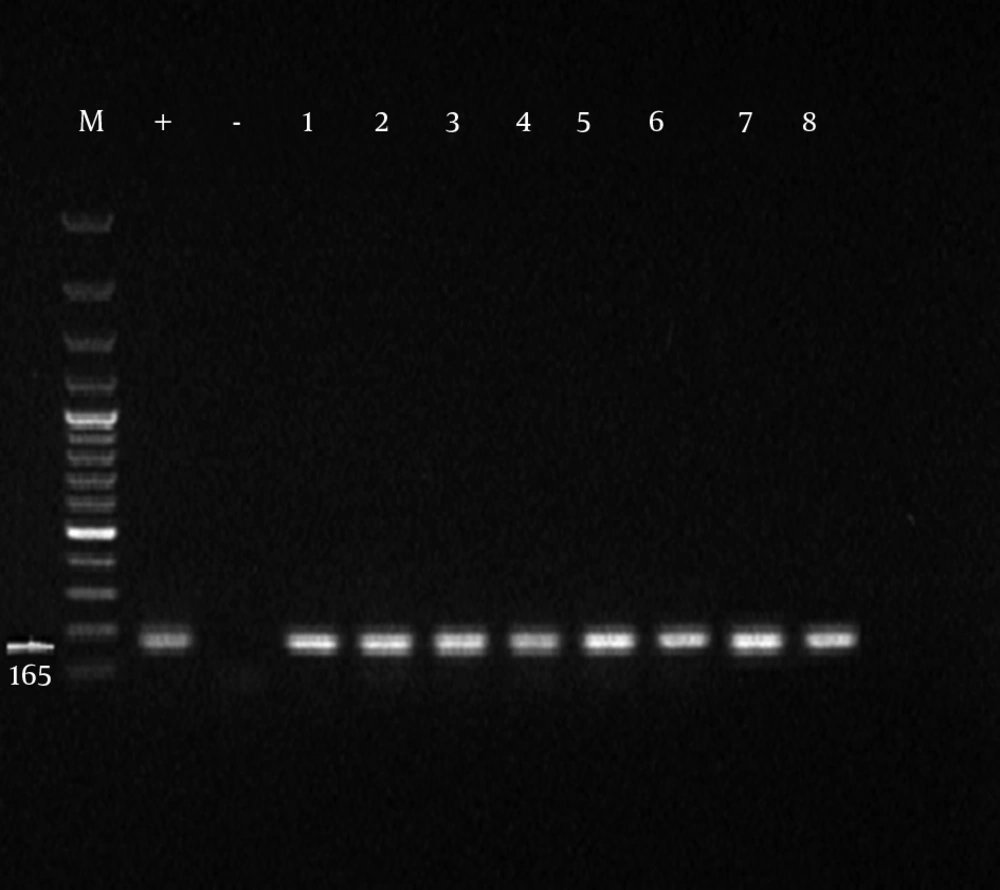
To perform PCR, the Master mix (Sinagen Company, Iran) was used, which contains Taq polymerase enzyme (0.5 IU/L), MgCl2 (4 mmol/L), and dNTPs (4 mM/L). Specific primers were used to identify the genus Mycoplasma and M. hominis (Tables 1 and 2) (11).
| Primer | Target Gene | Sequence | Length, bp |
|---|---|---|---|
| GSO | 16S rRNA | F: 5/-GGGAGCAAACAGGATTAGATACCCT -3/ | 163 |
| MGSO | R: 5/-TGCACCATCTGTCACTCTGTTAACCTC -3/ | ||
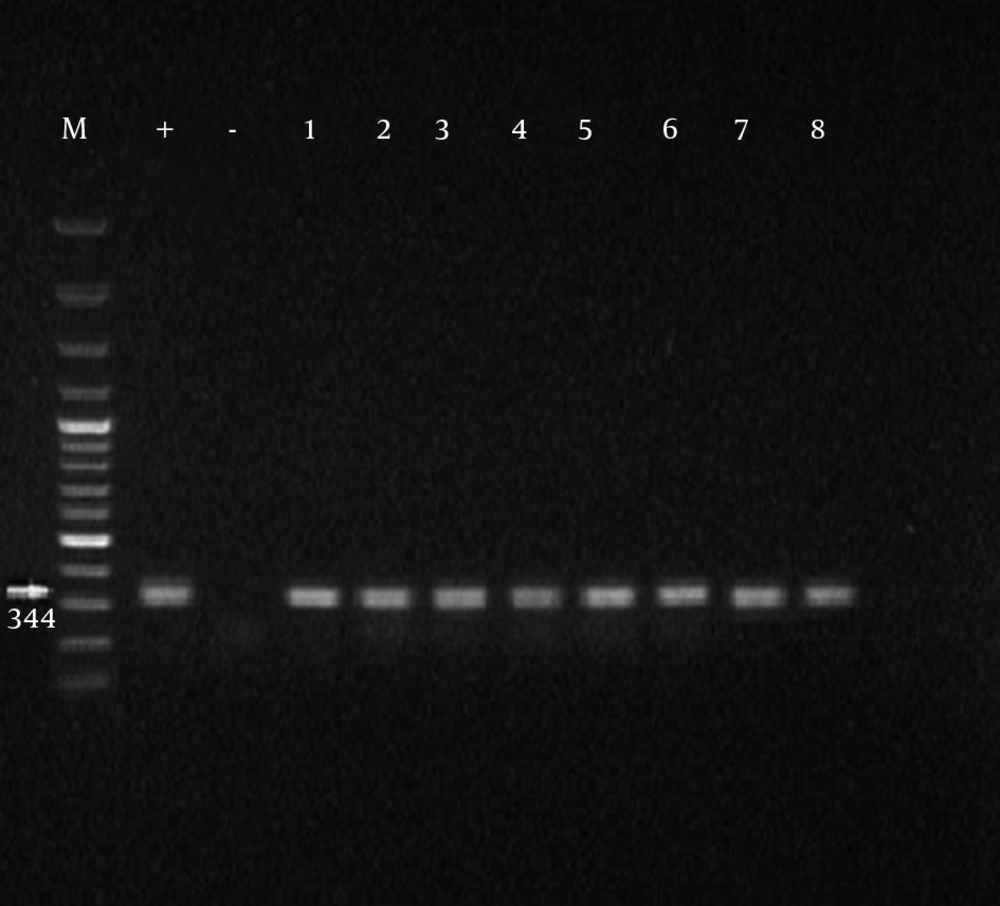
| RNA H1 | 16S rRNA | F: 5/-CAATGGCTAATGCCGGATACGC-3/ | 344 |
| RNA H2 | R:5/-GGTACCGTCAGTCTGCAAT-3/ |
| Stage | Temperature | Time | Cycle |
|---|---|---|---|
| Prog.1: Predenaturation | 94℃ | 6 | 1 |
| Prog.2: | 33 | ||
| Seg.1: Denaturation | 94℃ | 1 | |
| Seg.2: Annealing | 55℃ | 1 | |
| Seg.3: Extension | 70℃ | 1 | |
| Prog.3: Full Extension | 72℃ | 7 | 1 |
3.2. Sequencing
After purification, the PCR products were sent to the Bioneer Co, South Korea, to determine the nucleotide sequence. Bidirectional sequencing was performed with forward and reverse primers and finally, two sequences were obtained for each sample. Sequences multiple alignment was done using BioEdit software (available through http://www.mbio.ncsu.edu/RNaseP/info/programs/BIOEDIT/bioedit.html) by Clustal W method. MEGA 5 software was used to compare the samples nucleotide sequences and displaying similarities matrix in Excel. Using MEGA 5 software based on Neighbor-joining tree and with Bootstrap 1000, the phylogenetic tree of the samples was analyzed and drawn. Then the obtained sequence was compared with other sequences of M. hominis in NCBI gene bank site to ensure its specificity. Using the above software, the isolate similarity matrix of this study with isolates from rest of the world, recorded in the gene bank, was plotted. The phylogenetic tree of the samples was then drawn.
The PCR temperature program for amplification of target gene was performed using the specific primers based on the research protocol of Vosooghi et al. (10).
4. Results
Initially, the samples with positive results for Mycoplasma genus were separated and PCR was performed on them for identification of M. hominis. Formation of 344 bp band on an agarose gel indicated the positive strains of M. hominis. Of 100 obtained samples from infertile males, the presence of Mycoplasma genus was confirmed in 45 cases among which 33% were infected with M. hominis (Tables 3 and 4). From 100 obtained specimens from infertile females, the presence of Mycoplasma genus was confirmed in 43 cases of which 41.8% were infected with M. hominis (Figures 1 and 2).
| Genus Positive | Species Positive | Total Samples | |
|---|---|---|---|
| Male | 45 (45) | 15 (33) | 100 (100) |
| Female | 43 (43) | 18(41.8) | 100 (100) |
| Total | 88 44) | 33(37.5) | 200 (100) |
aData are presented as No. (%).
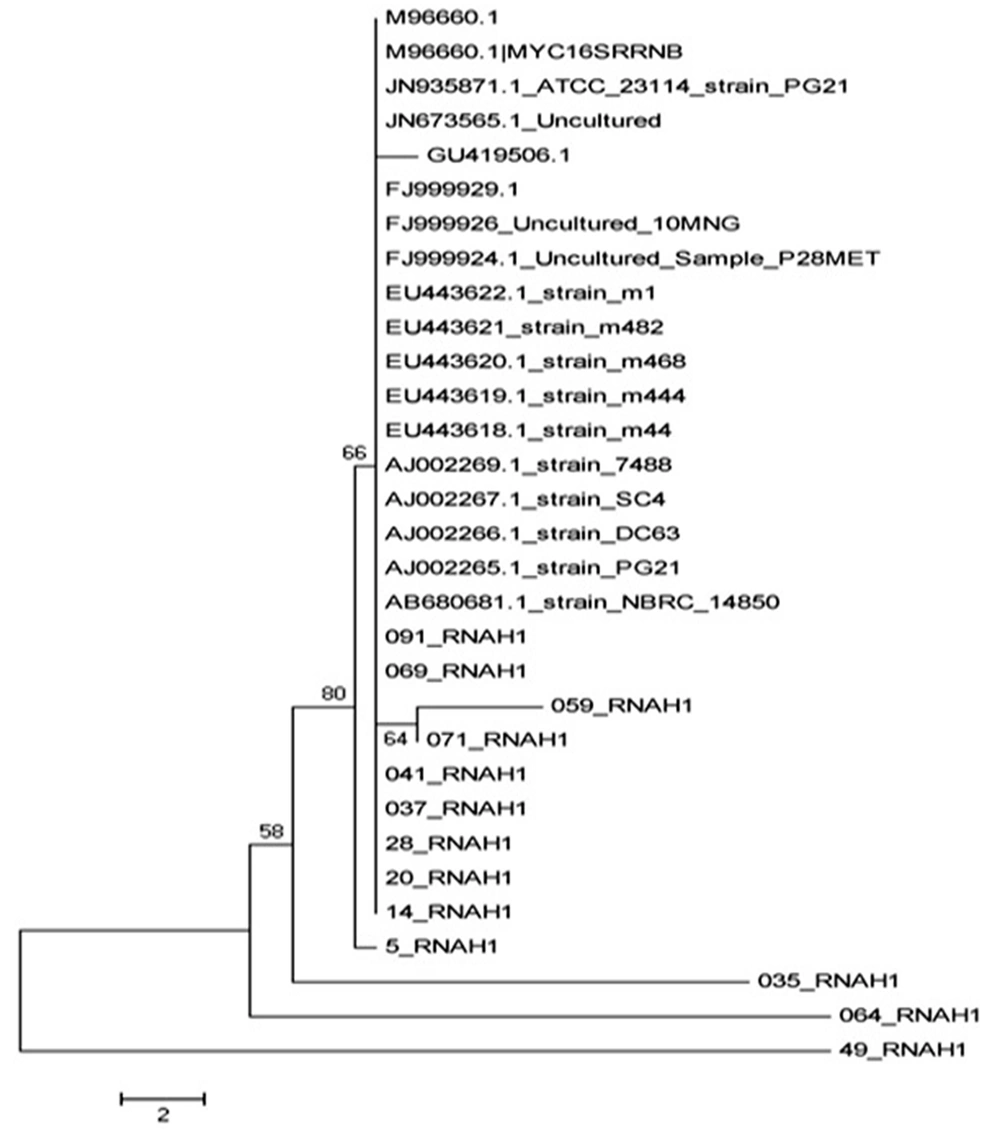
The phylogenetic tree was developed based on Neighbor-joining method using the MEGA5 software, and the corresponding file was saved as images, as can be seen in Figure 3.
| Access No. | JN935871 | GU419506 | JQ960910 | JN673565 | AJ002265 | AJ002266 | AJ002267 | AJ002269 | EU443618 | EU443619 | EU443620 | EU443621 | EU443622 | FJ999924 | FJ999926 | FJ999929 | M96660 | AB680681 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Country | USA | USA | USA | USA | DENMARK | DENMARK | DENMARK | DENMARK | RUSSIA | RUSSIA | RUSSIA | RUSSIA | RUSSIA | UK | UK | UK | CHINA | JAPAN |
5. Discussion
Mycoplasmahominis was isolated and identified as one of the infectious agents in urogenital secretions. Many studies have been done on Mycoplasma in Iran and around the world; however, such a research has not been done so far in Iran. In general, identification of Mycoplasma helps to understand the distribution and epidemiology of this infectious agent. urogenital infections have a key role in male infertility. Bacteria or its toxins bind to the sperm, stimulate immune system, and damage sperms in all anatomic areas of urogenital tract and reproductive system. Most of infertile males have a history of sexually transmitted diseases (STDs) such as gonococcal or nongonococcal urethritis. Moreover, the asymptomatic infected males will finally infect their partners and cause secondary couple infertility problems. Most of infertile males' wives have asymptomatic STDs, which might be related to their infected husbands (12). The first priority of this study was to determine the molecular identity of M. hominis as the causing factor of urogenital infections by using PCR. As PCR is a highly specific and rapid method for isolation of the factor, the obtained samples were not enriched in this study. Ahmadi et al. mentioned that the PCR is usually faster than conventional tests and can be used as a reliable method for isolation of Mycoplasma from urogenital specimens (9). Bacterial infection is one of the major causes of male and female infertility. Several bacteria genus are involved in infertility, the most common and most important of which are Mycoplasma species such as M. hominis that play an important role in developing infertility by creating infections without clinical symptoms that lead to not referring to the physician and the disease progression (13). In our study, the rate of infection with Mycoplasma genus (44%) were higher than the results of Ghazi Saeedi et al. study on first drops of urine (12%) and the prostatic secretions (14.6%) of males for isolation of Mycoplasma species (13). The contradictory results could be due to differences in the types of samples used for the experiments. Moreover, the samples used in this study were obtained from fertile males with urinary tract infection (14). In another study on semen samples of infertile males by culturing method, the M. hominis infection rate was 3.2%, which was much lower than that of our study (15). This difference might be due to the evaluation method of infection to isolate the Mycoplasma. PCR is much more accurate and sensitive than culture. In Ahmadi et al. research on semen samples of infertile males by PCR, the infection rate with M. hominis was 15.5% (9). The prevalence of urogenital infection with Mycoplasma in studies by Luki et al. in Canada, using cultivation method, was more than 50% (15), which was higher than our results (44%). The reason could be related to prevailing cultural and social factors and differences among countries. Freedom of sexual relationships and the number of sexual partners in these countries can be mentioned as reasons for the increased prevalence of Mycoplasma (17). In Vtani et al. research on females with vaginal infections by PCR, the isolation rate of Mycoplasma was 40.8%. (18), which was close to our results (44%). However, the results of Bakhshandenosrat et al. study on the prevalence of Mycoplasma in genital infection of females in Gorgan showed that Mycoplasma frequency was 12.8% and the prevalence of M. hominis was 7.7% (19), which were lower than that of our study. The low frequency of Mycoplasma in females of Gorgan was lower than expected, which might be due to the climatic conditions of the region. The results of alignment set, based on ATCC23114 strain sequence, showed that M. hominis isolates in this study were genetically classified in five distinct lineages with little similarity between isolates of the five lineages. The 069 and 091 isolates have a high genetic similarity with isolated in the gene bank, which have been geographically isolated in Japan, Denmark, Russia, and Britain, and were placed in a line. The isolates of 059, 071, 041, 037, 28, 20, and 14 had a high genetic similarity with each other but little similarity with other strains of gene bank and other isolated strains in this study. Other isolates of this study, including 035, 064, and 49, were placed in a lineage independent of all isolates in this study and the isolates in the gene bank. In fact, they can be registered as the native species of Iran in the gene bank. In general, this study showed that M. hominis could be considered as the most important bacterial factor of infertility in males and females in Kerman. In addition, the isolates identification suggests that regarding molecular structure and nucleotide sequence, Kerman isolates can be divided into two categories:
1) The first group with similarities with other isolates in Iran and the world in the gene banks
2) The second group as independent lineages with no phylogenetic affinities with any strains recorded in the gene bank
According to the results of this study, it is suggested that in case of urogenital infection with Mycoplasma, the couples should be concurrently and completely treated with appropriate antibiotics; moreover, public education regarding further adequate sanitation and necessary prevention measures should be considered. The rate of Mycoplasma genus in urogenital tracts of people not suffering from genital infections and infertility should be measured and their molecular identity in normal flora should be determined as well as analyzed.