1. Background
Renal biopsy is a well-established diagnostic modality for the assessment of kidney diseases in children. It can provide diagnostic precision and prognostic value and guide in therapeutic options for many renal diseases. A thorough knowledge of the epidemiology of diseases along with their clinicopathological correlations provides important information in clinical practice. Published renal registries indicate that there is variation in the epidemiology and spectrum of renal diseases in the pediatric age group in different geographical regions (1-6).
2. Objectives
We conducted the present study to evaluate the epidemiology of renal diseases in children in our set-up by studying their renal biopsies, indications, and histopathological spectrum.
3. Patients and Methods
We analyzed renal biopsies performed in our center from January 2008 to December 2013 in children ≤ 14 years of age. All the biopsies were performed by pediatric nephrologists under ultrasound guidance using 18-gauge renal biopsy needles. Two cores of the renal tissue were taken: one for light microscopy and one for immunofluorescence (IF) studies. Electron microscopy was not performed due to its non-availability. For light microscopy, 3-μm thick paraffin sections were stained for hematoxylin and eosin (H&E), periodic acid schiff (PAS), Jones’ methenamine silver (JMS), and Gomori’s trichrome (GMT) stains. The IF sections were stained using anti-human IgG, IgA, IgM, C3, and C1q antisera (DAKO, U.S.A.). Fibrinogen was used in selected cases of acute nephritis/rapidly progressive renal failure or in case of suspected vasculitis. Anti-human kappa and lambda light chain antisera were used only for suspected light chain involvement. Demographic evaluation included age, gender, clinical and histological diagnosis, serum creatinine (mg/dL), 24 hours’ urinary protein (g/24 hours), presence of microscopic hematuria, and hypertension. Hypertension was defined as blood pressure > 140/90 mm Hg and/or ongoing antihypertensive medication. The indications for renal biopsy comprised nephrotic syndrome (NS), acute nephritic syndrome (ANS), urinary abnormality (UA), and chronic renal failure (CRF). NS was defined as edema, nephrotic range proteinuria (> 40 mg/m²/h on timed sample and spot albumin to creatinine ratio > 2 mg/dL), and hypoalbuminemia (< 2.5 g/dL). ANS was defined as hematuria, hypertension, oliguria, edema, and reduced glomerular filtration rate. UA was considered as persistent non-nephrotic proteinuria with or without microhematuria. CRF was considered if the creatinine clearance was < 75 mL/min/1.73 m2. Remission was defined as urinary protein excretion < 4 mg/m2/h, nil or trace by dipstick on spot sample for 3 consecutive days. Steroid resistance (SR) was defined as failure to achieve remission after 4 weeks of daily therapy with oral prednisolone at a dose of 2 mg/kg/day. Steroid-dependent nephrotic syndrome (SDNS) was defined as 2 consecutive relapses within 14 days after the complete withdrawal of steroid or during the tapering phase of treatment. Steroid-dependent, frequently relapsing nephrotic syndrome (SDFRNS) was defined as 2 or more relapses in 6 months or ≥ 4 relapses in 12 months. IgM nephropathy (IgMN) was diagnosed when there was diffuse, global mesangial IgM staining on IF > +2 intensity in nonsclerotic glomeruli. Minimal change disease (MCD) was diagnosed in patients with proteinuria with little or no light microscopy or IF abnormalities. Mesangial proliferative glomerulonephritis (MePGN) was diagnosed when there were > 4 mesangial cells per mesangial region in > 80% of the glomeruli with no immunological involvement.
4. Results
Of the total 346 renal biopsies performed, 11 (3.17%) biopsies were inadequate, so 335 renal biopsies were considered for analysis. The mean age of the patients was 7.91 ± 3.04 years with a male preponderance (68.05%). The clinical and laboratory indications for renal biopsy were NS (46.22%), ANS (10.74%), UA (41.19%), and CRF (1.79%). NS (46.22%) was the most common indication, followed by UA (41.19%), ANS (10.74%), and CRF (1.79%). SDNS and steroid-resistant nephrotic syndrome (SRNS) accounted for 64.51% and 34.48% of the total NS. In NS, the most common glomerular disease was idiopathic MePGN (33.5%), followed by IgMN (21.2%), focal segmental glomerulosclerosis (FSGS) (12.2%), and MCD (12.2%). In SDNS, the most common glomerulopathy was MePGN (39%), followed by IgMN (20%) and MCD (18%). In SR, the most common glomerulopathy was FSGS (25.4%), followed by IgMN (23.6%) and MePGN (23.6%) (Table 1).
| Number of Subjects | MePGN | IgMN | FSGS | MCD | MPGN | MN | DPLN | C1qN | |
|---|---|---|---|---|---|---|---|---|---|
| Total NS | 155 (46.22) | 52 (33.55) | 33 (21.29) | 19 (12.26) | 19 (12.26) | 15 (9.67 | 8 (5.16) | 3 (1.94) | 1 (0.65) |
| SDNS | 100 (64.51) | 39 (39) | 20 (20) | 5 (5) | 18 (18) | 5 (5) | 7 (7) | 1 (1) | 1 (1) |
| SRNS | 55 (34.48) | 13 (23.63) | 13 (23.63) | 14 (25.45) | 1 (1.8) | 10 (18.18) | 1 (1.8) | 2 (3.63) | 0 |
a Abbreviations: C1qN: C1q nephropathy; DPLN: diffuse proliferative glomerulonephritis, FSGS: focal segmental glomerulosclerosis, IgMN: IgM nephropathy, MCD: minimal change disease, MePGN: mesangial proliferative glomerulonephritis, MN: membranous nephropathy, MPGN: membranoproliferative glomerulonephritis, SDNS: steroid-dependent nephrotic syndrome, and SRNS: steroid-resistant nephrotic syndrome.
b The values are presented as No (%).
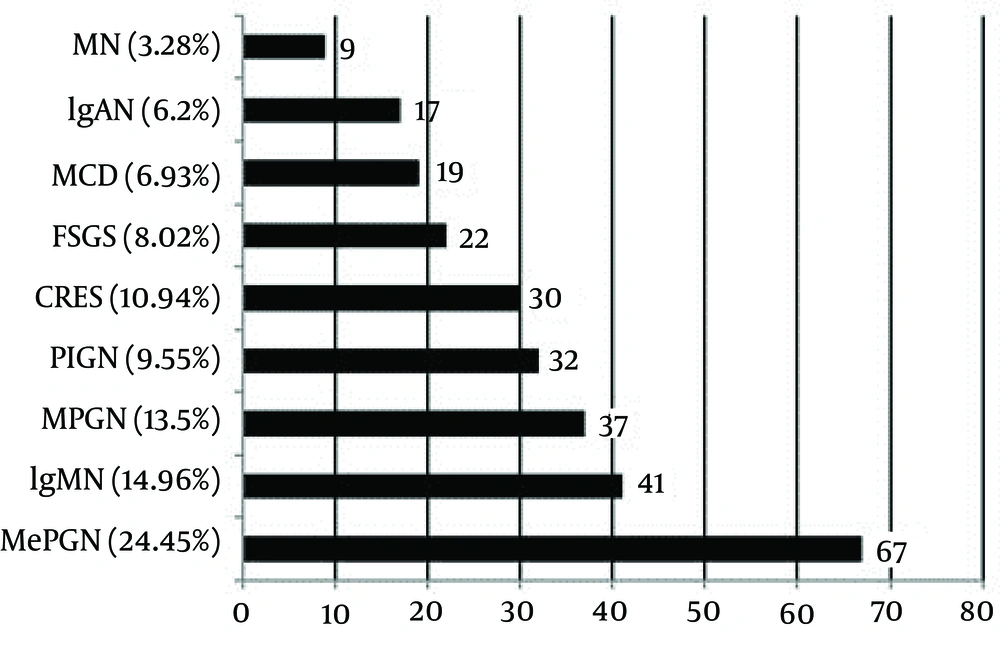
Primary GN (81.79%) was the predominant GN, and secondary GN constituted 16.12% of the cases. Primary GN included MePGN, MCD, IgMN, IgA nephropathy (IgAN), FSGS, membranoproliferative glomerulonephritis (MPGN), membranous nephropathy (MN), crescentic GN, and post-infectious glomerulonephritis (PIGN). Secondary GN was classified into lupus nephritis (LN), hemolytic uremic syndrome (HUS), amyloid, and hypertensive nephropathy (HTN). Tubulointerstitial nephritis was reported in 2.08% of the patients. The most common histological pattern of primary GN was MePGN (24.45%), followed by IgMN (14.96%), MPGN (13.5%), PIGN (9.55%), crescentic GN (10.94%), FSGS (8.02%), MCD (6.93%), IgAN (6.2%), and MN (3.28%) (Figure 1).
MePGN: mesangial proliferative glomerulonephritis, IgMN: IgM nephropathy, MPGN: membranoproliferative glomerulonephritis, PIGN: post-infectious glomerulonephritis, CRES GN: crescentic glomerulonephritis, FSGS: focal segmental glomerulosclerosis, MCD: minimal change disease, IgAN: IgA nephropathy, and MN: membranous nephropathy.
Secondary GN was reported in 54 (16.12%) patients. The most common lesion was LN (7.76%), followed by HUS (6.26%), HTN (1.19%), and amyloidosis (0.89%).
5. Discussion
This report provides information on the histopathological pattern and epidemiology of renal diseases in children in India. To our knowledge, this is the first systematic review of histological data of children suffering from renal diseases in India. NS was the most common indication for renal biopsy in the children in that it comprised 46.2% of the total biopsies in our study. A similar observation has been reported in different series from Europe and Asia (6-10). In NS, MePGN (33.5%) was the most common histological lesion, followed by IgMN (21.2%), FSGS (12.2%), and MCD (12.2%). In SRNS, FSGS (25.4%) and IgMN (23.6%) were the common lesions. In their overall study of nephritic children in North India, Kumar et al. (11) reported that MCD was the most common lesion in those under 8 years of age and that FSGS was the most common NS. Mubarak et al. (12) reported MCD as the most common NS, followed by FSGS, in childhood NS in Pakistan. It is consensual not to perform renal biopsy in children with a recent diagnosis of NS without the following criteria: arterial hypertension; gross hematuria and/or marked elevation in serum creatinine; normal complement levels; and no external symptoms or signs suggestive of secondary GN (3). We usually perform renal biopsies in children with SRNS, SDNS, and SDFRNS. Children with MCD usually do not undergo renal biopsy at our center; this may be the reason for the high incidence of MePGN and IgMN compared to MCD in our study. Along the same lines, Pio et al. also reported the rising prevalence of MePGN and IgM deposits for the same reason (3). MCD with IgMN comprised one-third of the NS in our study. The IgMN morphology can vary from normal-appearing glomeruli to mesangial hyperplasia with or without segmental or global sclerosis. In the present study, IgMN comprised MePGN (73.17%), FSGS (14.63%), and MCD (12.19%). IgMN is usually steroid-resistant and has a poor prognosis compared to similar lesions without IgM deposits (2, 13-15). Our previous published study reported that IgMN was observed in 11.9% of the biopsies, with the most common morphology being MePGN followed by MCD and FSGS (16).
Primary GN (81.8%) was the most common histology in our study. A similar observation has been reported in other studies (1, 3, 10). The most common primary GN was MePGN in our study, while IgAN was reported as the most common cause of primary GN in other Asian and European series (1, 2, 17). The low prevalence of IgAN in our study (5.07% of primary GN) is due to the low rate of renal biopsies performed in children with UA. Vanikar et al. (18) reported an overall incidence of primary IgAN in patients with nephrotic/nephritic syndrome of 16.2% in western India, more common in young males in second/third decade of life. The Italian national registry demonstrated that 50% of biopsies were performed for isolated hematuria and non-nephrotic proteinuria (1, 10). This shows that difference in lesions depends on the selection criteria for renal biopsy rather than difference in frequency. In the present study, secondary GN was reported in 54 (16.1%) patients. The most common lesion in secondary GN was LN (7.76%), followed by HUS (6.26%), HTN (1.19%), and amyloidosis (0.89%). Chiming in with our results, LN was the most common secondary GN observed in other studies too (2, 3, 6). In pediatric patients, the indication and histopathological findings of renal biopsies are similar in the same region; however, they differ from those in Western and even Asian countries. As was mentioned, the difference in lesions also depends on the selection criteria for renal biopsy. The present study provides data on the epidemiology of renal diseases in Indian children and will be helpful for building up a national registry and devising therapeutic guidelines in the future.