1. Background
High flux membranes have been applied for hemodialysis (HD). High flux dialysis membrane is defined as a β2-microglobulin clearance of over 20 mL/min (1, 2). It is able to remove low molecular weight proteins between 20000 and 30000 Da. However, smaller molecules of uremic toxin connect albumin (molecular weight = 66,000) are difficult to separate protein-bound toxins from albumin, because albumin molecular weight is high. Indoxyl sulfate (IS) is one of protein-bound toxins (3) known to release reactive oxygen species (ROS) to the blood stream and causes some dialysis-related complications. Therefore, it might be better if IS is dissociated from albumin and excluded during HD therapy. Yamamoto et al. demonstrated in vitro that dilution and pH change of dialysate facilitate IS dissociate from albumin (4). The predilution mode can dilute the blood before hemodia filter in on-line HDF therapy. This method also facilitates the dissociation of IS from albumin by dilution and removal of dissociated IS (molecular weight = 213) by diffusion.
Recent basic and clinical researches have revealed that hydrogen is an important physiological regulatory factor with antioxidant, anti-inflammatory and anti-apoptotic protective effects on cells and organs (5). A water electrolysis technique has been developed (6) and enabled to apply high dissolved hydrogen solution to clinical HD system. Use of dialysate with high dissolved hydrogen may prove to be a novel approach for amelioration of dialysis-related complications such as intradialytic hypotension (7).
2. Objectives
We made dialysate with high dissolved hydrogen. We examined in vitro whether IS dissociated from albumin in simulated HD system using this dialysate.
3. Materials and Methods
3.1. Characteristic of Dialysate With High Dissolved Hydrogen
We obtained high dissolved hydrogen solution by ion water conditioner (NIHON TRIM, Japan). To determine the characteristics of this solution, we used oxidation-reduction potential (ORP) meter (YK- 23RP, Mother Tool, Japan), and hydrogen monitor (ENH- 1000, TRUSTLEX, Japan). Sampling time was set at 0 minute as baseline, 30 minutes, 60 minutes, 120 minutes, 180 minutes, 240 minutes, 360 minutes, 12 hours and 24 hours after making this solution. Measurements were repeated five times.
3.2. How to Make Conventional Dialysate and Dialysate With High Dissolved Hydrogen
We made conventional dialysate and the dialysate with high dissolved hydrogen using reverse osmosis (RO) water and high dissolved hydrogen solution, respectively and diluted 35 times with concentrated dialysate (KINDARY AF- 2, FUSO, Japan). pH, partial pressure of carbon dioxide (PCO2), oxygen (PO2) and HCO3 of conventional dialysate and high dissolved hydrogen dialysate were determined using i-STAT (300F, FUSO, Japan).
3.3. Examination of Indoxyl Sulfate Separated From Albumin Using Dialysate With High Dissolved Hydrogen
We obtained 100 L wasted dialysate after continuous ambulatory peritoneal dialysis (CAPD) in a patient. We concentrated CAPD solution 100 times by extracorporeal ultrafiltration method using FB-190E (CTA, low-flux, NIPRO, Japan). One hundred mL of this solution was mixed with 100 mL of conventional dialysate or dialysate with high dissolved hydrogen dialysate. Experiment was set at blood flow rate of 100 mL/min, filtration flow of 5 mL/min and dialyzer FB-70E (CTA, low-flux, NIPRO, Japan) as a filter to separate free IS and albumin-bound IS. We obtained 100 mL filtrated dialysate. The concentration of IS was determined by high performance liquid chromatography. Free fractions (FR) were calculated as below formula (4).

CB: initial concentration of blood side
CF: concentration of filtration side
3.4. Statistical Analysis
Statistical analyses were conducted using Stat View (Version 5, SAS, USA). Continuous variables were described as mean ± standard deviation (SD) and compared with repeated measure ANOVA of Tukey-Kramer or Student’s t-test as appropriate. P value less than 0.05 or 0.01 was regarded as significant.
4. Results
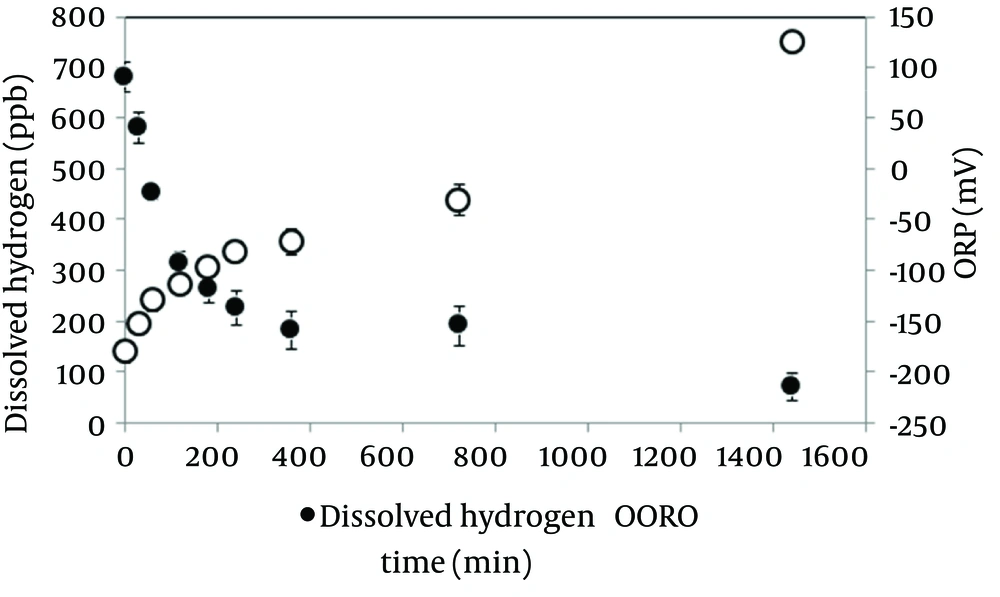
Dissolved hydrogen concentration and ORP in the electrolyzed solution in the high dissolved hydrogen solution. Changes in dissolved hydrogen and ORP after making the solution are shown in Figure 1. Dissolved hydrogen decreased half values compared at baseline in 120 minutes. Time course of ORP gradually increased. These results suggested that the half-life of hydrogen water was 120 minutes; the experiment was performed using electrolyzed solution in the high dissolved hydrogen within 30 minutes after making.
Solute characteristics of both dialysate profiles are shown in Table 1. Dissolved hydrogen showed significantly higher than conventional dialysate (P < 0.01). Comparison between conventional dialysate and high dissolved hydrogen in the initial and filtrated concentrations of albumin IS and FR is shown in Table 2. FR of conventional dialysate showed 35.4 ± 4.0%. FR of high dissolved hydrogen showed 41.7 ± 2.6%. FR of high dissolved hydrogen was significantly higher than conventional dialysate (P < 0.05).
| Variables | Conventional Dialysate | High H2 Dialysate | P Value |
|---|---|---|---|
| pH | 7.59 ± 0.18 | 7.60 ± 0.06 | ns |
| PCO2, mmHg | 27.6 ± 8.92 | 27.3 ± 2.87 | ns |
| PO2, mmHg | 144.6 ± 14.57 | 134 ± 20.22 | ns |
| HCO3, mmol/L | 25.4 ± 1.6 | 26.7 ± 1.0 | ns |
| Dissolved H2, ppb | 0 | 699.8 ± 179.9 | < 0.01 |
a All of the values are presented as Mean ± SD.
| Variables | Conventional Dialysate | High H2 Dialysate | P Value |
|---|---|---|---|
| Initial concentration of albumin, μg/mL | 7569 ± 175 | 7527 ± 310 | ns |
| Concentration of filtrated albumin,μg/mL | 39.1 ± 3.6 | 38.8 ± 5.4 | ns |
| Initial concentration of IS,μg/mL | 8.3 ± 0.6 | 8.4 ± 0.5 | ns |
| Concentration of filtrated IS,μg/mL | 2.9 ± 0.4 | 3.5 ± 0.1 | < 0.05 |
| FR of IS, % | 35.4 ± 0.4 | 41.7 ± 2.7 | < 0.05 |
a All values are presented as Mean ± SD.
b Abbreviations: FR, Free Fractions; IS, Indoxyl Sulfate.
5. Discussion
Protein-bound uremic toxins, especially IS, induce vascular inflammation, endothelial dysfunction and vascular calcification, which may explain the relatively poor prognosis of chronic kidney disease and patients under dialysis (8). Plasma IS was associated with first heart failure event in patients on HD (9). HD using a high-flux membrane cannot efficiently remove the protein-bound uremic toxins because of their high albumin-binding property. Especially, IS showed high protein-binding ratios (more than 95%) and low reduction rates by HD (< 35%). Removal of IS can be improved to some extent by increasing the diffusion of free forms with super-flux membrane HD and/or HDF (10). IS is largely albumin bound and inhibits drug protein biding (11-14). Furthermore, this compound accelerates the progression of glomerulosclerosis in the rat (15, 16). If IS dissociates from albumin, it can easily remove by diffusion. Thus, IS removal is expected as new dialysis techniques. We focused on dialysate with high dissolved hydrogen and examined characteristics in this solution.
The level of dissolved hydrogen values was halved by two hours after obtaining electrolyzed water. These results suggested that the solution should be used immediately after preparation. Anti-oxidation has been confirmed using electrolyzed water by animal experiments (17-20). Furthermore, hydrogen gas has been reported to exhibit antioxidant properties. Electrolytic water containing hydrogen attracted more attention recently (21). Consumption of water with dissolved hydrogen produced by electrolysis by ad libitum drinking has the potential to ameliorate ischemia-induced cardio-renal injury in chronic kidney disease model rats (22). Using hydrogen-enriched solutions could ameliorate oxidative stress and albumin redox during HD (23).
Therefore, we focused on IS and developed a dialysate containing high dissolved hydrogen. We examined in vitro whether it is possible to separate IS from albumin. IS can be easily separated by dilution. This study demonstrated that hydrogen water promoted IS to dissociate from albumin during HD therapy. More beneficial effects would be expected in the combination of predilution mode on-line HDF with hydrogen water. More clinical studies are necessary on this issue. Dialysate with high dissolved hydrogen can significantly dissociate IS from albumin compared to conventional dialysate. Using this solution, high efficient IS removal was proved in vitro.