1. Introduction
Hemophagocytic syndrome (HS) is a clinical group of entities, biological and histological manifestations secondary to activation and proliferation of macrophages, which is responsible for blood cell phagocytosis (1). It can be primary or secondary to a lot of pathologies such as infections, cancer or auto-immune disease (2, 3). Despite its scarcity, some cases of HS in association with systemic auto-immune diseases have been reported in Africa (4, 5). However, the occurrence of this syndrome during pauci-immune vasculitis is less noticed. We reported here a case of HS complicating microscopic polyangitis presented by crescentic glomerulonephritis.
2. Case Presentation
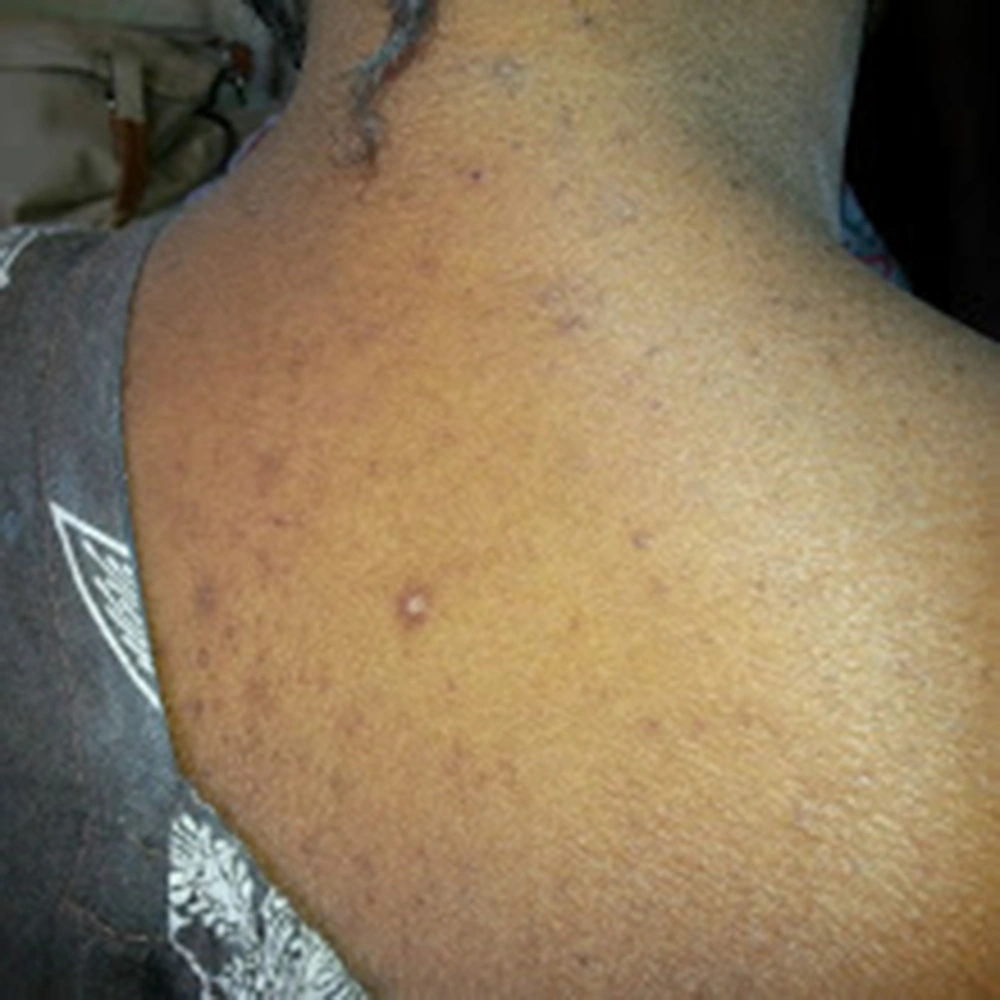
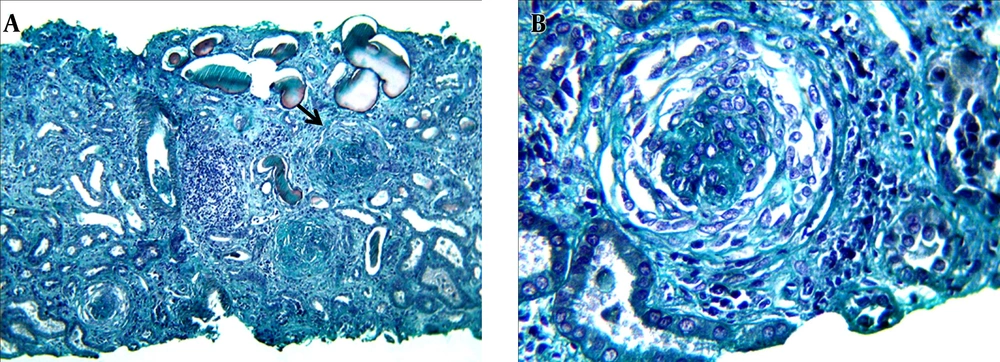
A 22-year-old female patient originated from Dakar/Senegal, with no known medical history presented in May 2014 with progressive low limb edema and puffy face. She was admitted in nephrology department 2 weeks after the beginning of symptoms and treated by only free-salt diet. On admission, physical examination found high blood pressure at 150/100 mmHg, glomerular range proteinuria (++++) on urine dipstick with no hematuria. On physical examination, hyperchromic diffuse punctilious purpura skin lesions predominant on the trunk, the neck and the upper thigh (Figure 1) were found. Paraclinical investigations revealed a nephrotic syndrome with 24 hours proteinuria of 4.29 grams, hypoalbuminemia of 19.4 g/L and hypoproteinemia of 56 g/L. Serum creatinine was 60.9 mg/L and urea 1.62 g/L. Full blood count (FBC) showed normochromic and normocytic anemia of 9.9 g/dL hemoglobin, 4700/mm3 WBC and 434103/mm3 platelets. Auto-immunity investigations revealed negative results for anti DNA and anti Sm antibodies. However, anti SSA/Ro, anti-SSB/La and anti U1 RNP were strongly positive meaning a possible Sjogren syndrome. Anti- neutrophil cytoplasmic antibodies (ANCA) was positive with a peri-nuclear type fluorescence (p-ANCA), specific to myeloperoxidase (MPO). HBV, HCV and HIV serology had negative results as well as syphilis (TPHA-VDRL). The kidneys were at good size and differentiation. In optic microscopy (OM), renal biopsy performed after blood pressure control showed a crescentic glomerulonephritis (GN) with circumferential cellular and fibrous proliferation affecting 85% of glomeruli. There was also tubular and interstitial involvement with moderate edema and inflammation together with mild endarteritis fibrosa (Figure 2). Immunofluorescence (IF) was not performed.
Diagnosis of microscopic polyangitis with renal and skin involvement probably associated with a secondary Sjogren syndrome was retained. The patient presented a five score of 2 with 24 hours proteinuria, more than 2 grams and impaired GFR. She underwent dialysis and received angiotensin converting inhibitor (ACE) and furosemide before renal biopsy. After renal biopsy, we added pulses of methylprednisolone at dose of 15 mg/kg/day for 3 days followed by oral prednisolone of 1 mg/kg/day and pulses of cyclophosphamide of 700 mg/m2 every 15 days for the first 3 pulses and every 21 days thereafter.
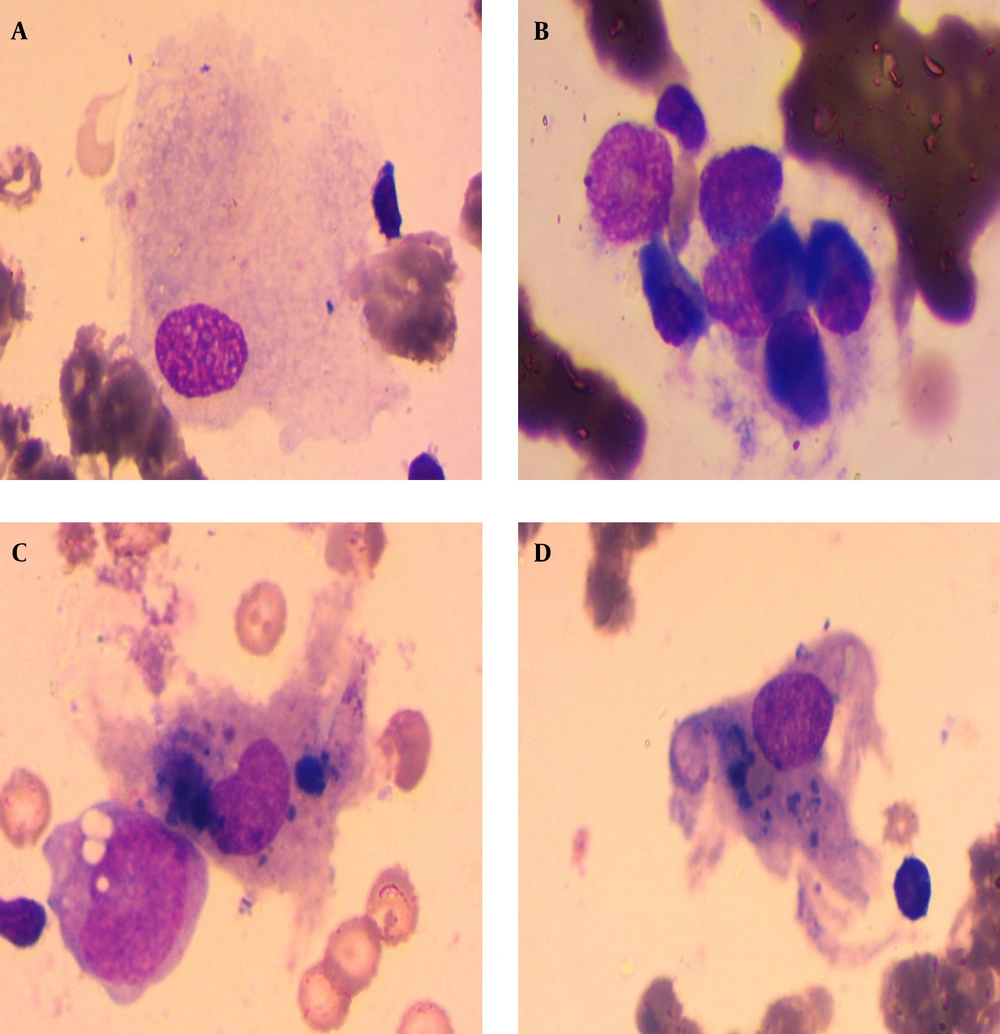
At the 5th month of treatment while she had just received her last pulse of cyclophosphamide, she was admitted with impaired consciousness and fever. Physical examination found obnubilation with no neurological focal sign. There were no sign of meningeal irritation. Cardiac examination found regular tachycardia. Lung auscultation was normal. Blood pressure (BP) was 135/85 mmhg and temperature 38.5°C. The following days were marked by worsening of the patient consciousness with a Glasgow coma scale of 9/15 and a hemorrhagic syndrome with melena, purpura skin lesions and gingival bleeding. Malaria tests had negative results. Complementary investigations revealed CRP of 96 mg/L, central pancytopenia with severe anemia 5.1 g/dL, neutrophillic leukocytopenia with 2000 leucocytes/mm3 and neutrophiles 700/mm3, thrombocytopenia 47000/mm3, and reticulocytes 67000/mm3. Natremia and kaliemia were normal. Other paraclinical findings showed increased ferritinemia at 4692 (23 times more than the normal range), LDH 468 IU/L (twice the normal) and hypertriglyceridemia 2.52 g/L (1.5 times the normal). Serum calcium and phosphorus were within normal ranges. Due to these clinical and biological findings, a bone marrow aspiration was performed, which found medullary invasion by macrophages with signs of hemophagocytosis (Figure 3).
Diagnosis of HS complicating a microscopic polyangitis was retained and methylprednisolone pulses started. We noticed apyrexia on the first day, improvement of consciousness and general condition in the following days. We also noticed progressive normalization of different biological parameters in the following days and weeks. The patient was under hemodialysis after follow-up of about 9 months with stable clinical state.
3. Discussion
HS is a clinical and pathological entity characterized by non-specific activation of macrophages leading to hemophagocytosis of blood cells and different tissue infiltration (bone marrow, spleen, lymph nodes, liver, etc.) (1). These activated macrophages secrete proinflammatory cytokines (IL-1, IL-6 and TNF-α) responsible of different nonspecific clinicobiological manifestations observed during this disease (6, 7). Clinical and biological manifestations of HS are nonspecific. However, their association should make the clinician think about it and do a bone marrow aspiration or an organ biopsy (liver or lymph nodes) to confirm it. Indeed, isolating macrophages and hemophagocytosis on bone marrow aspiration and histology remains a necessary condition to make the diagnosis of HS. Several criteria have been proposed in the literature (8, 9) for the diagnosis of HS, among them those of Henter in 2004 (10) validated by the histiocyte society. These require the presence of 5 criteria of 8 as found in our patient. She had fever, bicytopenia, hypertriglyceridemia, hyperferritinemia and bone marrow hemophagocytosis. However, these criteria are only validated in primary or pediatric HS and in extrapolation for adult and secondary HS (8). The study group on HS of the hystiocyte society is working on recommendations for diagnosis and management of HS in adults (6). Regarding etiologies, HS can be primary in relation to genetic abnormalities often discovered in children, or secondary to different infectious (bacterial, viral and parasitic), paraneoplastic or autoimmune pathologies (2). HS is differently described with auto-immune and inflammatory systemic diseases. Karras et al. (2) noted that the review of the 8 largest series of HS (with a total of 306 patients) concluded that the disease is secondary to a systemic auto-immune disease in 7.2% of cases. In more recent study, Fukaya et al. (11) found HS prevalence of 3% in 1040 patients admitted for a systemic auto-immune or inflammatory disease over 10 years in Japan. In West Africa, HS is scarcely reported. Some cases have been described in the last few years (4, 5). According to a literature review conducted by Atteritano et al. (3), the association between HS and autoimmune systemic diseases was found to be in descending order as follows: juvenile idiopathic systemic arthritis, lupus, Still’s disease, Kawasaki disease, dermatomyositis, rheumatoid arthritis, periarteritis nodosa, sarcoidosis, Sjogren syndrome, ankylosing spondylitis, Behcet disease, sharp syndrome and Wegener granulomatosis. The presentation of HS during microscopic polyangitis, an MPOANCA positive vasculitis, is scarcely described. Atteritano et al. (3) in their exhaustive review did not report any case. Some cases have been described though. Kumakura et al. (12) reported HS in a patient with MPO-ANCA positive vasculitis. Unlike the case of our patient, vasculitis was associated with systemic sclerosis. More recently, HS in a female patient with MPO-ANCA positive pauci-immune vasculitis overlapping Goodpasture syndrome has been reported (13). Similar to our patient, there was pancytopenia and elevated serum ferritin. Bone marrow aspiration found images of phagocytosis like our patient. This observation is to our knowledge the first case providing the association of MPO-ANCA positive pauci-immune systemic vasculitis and HS in West Africa. This is explained by diagnostic limitations of vasculitis that are not available in daily practice like IF. On the other hand, non-specific clinical and biological manifestations of HS suggest infectious diseases at first (malaria, typhoid and paratyphoid fever, tuberculosis, retroviral infection) once an acute fever occurred in our context. Nevertheless, until then it remains difficult to provide evidence of direct cause-effect relationship between vasculitis and HS in our patient. Indeed, the latter can be also due to auto-immune systemic diseases and immunosuppression treatments or even intercurrent infection secondary to immunosuppression caused by the same treatments. In our patient, we were not able to conduct extensive investigations regarding different infectious agents frequently occurred in HS, especially in patients receiving immunosuppression therapy for systemic diseases (14, 15) such as CMV, Herpes simplex virus and EBV. We managed HS in our patient with steroids boluses. In fact, the underlying disease influences the therapeutic choice. The use of steroid boluses with or without other immunosuppression tends to be the first therapy by many authors (1, 11, 16-18). However, some authors recommend the use of etoposide (VP16) to treat HS in emergency (defined by the existence of at least one organ failure), while etiologic investigations are underway (7).
HS is a clinico-pathological syndrome, which can compromise the prognosis. It can be secondary to systemic auto-immune or inflammatory diseases. Its presentation during pauci-immune vasculitis is rarely reported, especially in Africa. Therefore, our observation is an illustration of the reality of this association in our context. This should help physicians consider it in case of acute fever with cytopenia, especially in the context of systemic disease.