1. Background
Percutaneous nephrolithotomy (PCNL) is a common method of removing renal stones. Although renal parenchymal damage occurs during this procedure, it has been demonstrated that renal parenchymal function preserved after PCNL (1). This operation may be performed under general or spinal anesthesia; spinal anesthesia is as effective and safe as general anesthesia (2). Although this is a safe procedure, it is not recommended in cases using room air to opacify the collecting system (3). Postoperative pain is less intense after minimally invasive surgeries than open surgery but these surgeries, for example, PCNL are not pain-free; however, several attempts like using epidural analgesia, patient controlled analgesia and local anesthetics or opioid infiltration (4-6) have been performed. Among local anesthetics, although low-dose bupivacaine is commonly used with proper outcome, the use of high-dose bupivacaine may lead to cardiovascular side effects (7). Ropivacaine is another local anesthetic agent structurally similar to bupivacaine because of having S (-) enantiomer compounds, but without cardiovascular complications as well as higher sensory-motor blocking (8, 9). Extensive researches have been performed on the clinical efficacy of ropivacaine on relieving labor pain and reducing postoperative pain in both children and adults (10-12). In total, for analgesic effect and cardiac complication, ropivacaine has a higher efficacy compared to other similar anesthetics such as bupivacaine (13). Postoperative analgesic effects of intravenous or subcutaneous wound infiltration of opioids have been demonstrated in various urological surgeries (5, 14); however, the beneficial effect of ropivacaine on postoperative pain relief in urological surgeries such as tubeless percutaneous nephrolithotomy (PCNL) has remained uncertain.
2. Objectives
As general anesthesia is commonly used for this procedure, it seems that using local anesthetics, intensity and duration of analgesia may be improved. Hence, the present study aimed to assess the efficacy of ropivacaine on postoperative pain severity in patients undergoing tubeless PCNL procedure.
3. Patients and Methods
Following the approval of university ethics committee, this randomized double-blinded clinical trial was performed on 55 patients aged 15 to 60 years undergoing tubeless PCNL surgery in the urology ward of Sina Hospital, a university affiliated hospital of Tehran university of medical sciences. After the research was approved by the ethics committee of Tehran University of medical sciences, registered at IRCT under number IRCT201307153773N8. The exclusion criteria were body weight lower than 40 kg, presence of excessive surgical punctures on skin during PCNL (increased risk of drug toxicity) or presence of ureteral or urinary tract stones remaining in surgical zone needing higher doses of analgesic. After including eligible patients, informed consent was obtained from them and randomly assigned to two intervention and placebo groups using block randomization sampling method. Initially, all patients were pre-medicated by fentanil 1 µg/kg plus midazolam 0.02 mg/kg and then anesthesia was induced by thiopental 5 mg/kg plus atracurium 0.5 mg/kg; the patients were then intubated. Anesthesia was maintained by isoflurane and oxygen 100%. After the administration of anesthesia, patients were laid on the operating table in the supine position, they were placed in the lithotomy position and a 22 F cystoscope inserted transurethrally and an open-ended 6F ureteral catheter was advanced up to the kidney with a stone. Then, the patients were placed in the prone position. Under the guidance of fluoroscopy, radiopaque agent was delivered through ureteral catheter to opacify the pelvicalyceal system. An 18 G Shiba needle was advanced into the calyx through which maximal number of stones could be retrieved with minimal risk of bleeding. After the observation of urine outflow through the needle, guidewire was delivered through the needle into the pelvicalyceal system. The skin was incised with 20 G scalpel. Over the guidewire, 6F ureteral catheter, co-axial or one shut dilators were advanced to dilate the access tract. Over the dilator, a 30 F Amplatz dilator was used. The pelvicalyceal system was entered with a 25 F nephroscope. Stones were either extracted by forceps or fragmented with pneumatic lithotripter and extracted with a forceps. If necessary, for complete stone clearance, more than one entry was achieved. At the end of the operation, the nephrostomy tube was withdrawn. After ensuring lack of remnants of stone by fluoroscopy, the intervention group instilled with 30 mL of ropivacaine 0.2% at surgical zones including the renal puncture site (10 mL), nephrostomy tract (15 mL) and skin (5 mL) by a Nelaton catheter 8F. The placebo group received only 30 mL of isotonic normal saline with the same protocol. Twenty minutes before transferring patients to the recovery room, both groups received 1 g Apotel intravenously. The patients received Apotel three times a day. At the request of the patient due to feeling pain, meperidine (0.5 mg/kg) could be administered. Visual analogue scale (VAS) for assessing pain severity (0 - 10 Numeric Pain Rating Scale) and peak expiratory flow (PEF) (for assessing person’s ability to breathe out air) (15) were measured 4 and 6 hours after completing the procedure. Moreover, the amounts of opioids or analgesics administered within 6 hours after the operation were recorded.
3.1. Data Analysis
Results were presented as mean ± standard deviation (SD) for quantitative variables and summarized by absolute frequencies and percentages for categorical variables. Categorical variables were compared using chi-square test or Fisher’s exact test when more than 20% of cells with the expected count of less than 5 were observed. Continuous variables were compared using one-way analysis of t test and/or non-parametric Mann-Whitney test when data had not normal distribution. The trend of changes in the values of study endpoints was assessed using repeated measure ANOVA test. All statistical analyses were performed using SPSS software (version 21.0, SPSS Inc., Chicago, Illinois). All statistical tests were two-sided and differences with probability values < 0.05 were considered statistically significant.
4. Results
In total, 25 patients (mean age of 48.20 ± 13.53 years, 60.0% male) received ropivacaine as the case group and 30 patients in the placebo group (mean age of 47.20 ± 14.93 years, 70.0% male) received normal saline. The two groups were similar in sex distribution (P = 0.272), average age (P = 0.713) as well as other baseline parameters including mean weight, mean height, mean body mass index, history of cardiovascular disorders and hypertension (Table 1). Regarding intraoperative characteristics (Table 2), there were no significant differences in the number of holes on the skin for entering the catheter (intercostal or subcostal) or history of PCNL between the two groups. However, the frequency of smoking was higher in the placebo group. The two groups were similar in the mean operation duration, administration of analgesics or opioids during recovery time, number of analgesic request during 6 hours after the operation and mean doses of pethidine or morphine administered during 6 hours after the operation (Table 2). The groups were also similar in prevalence of residual kidney stone after the operation as well as the prevalence of horseshoe kidney.
| Item | Ropivacaine (n = 25) | Bupivacaine (n = 30) | P Value |
|---|---|---|---|
| Male Gender | 25 (60.0) | 21 (70.0) | .272 |
| Age, y | 48.20 ± 13.53 | 47.20 ± 14.93 | .713 |
| Weight, kg | 75.66 ± 13.69 | 75.47 ± 19.99 | .952 |
| Height, cm | 165.00 ± 9.37 | 168.63 ± 9.91 | .052 |
| Body Mass Index, kg/m2 | 28.96 ± 5.38 | 26.96 ± 5.81 | .063 |
| History of Heart Disease | 5 (20.0) | 5 (16.7) | .822 |
| History of PCNL | 1 (4.0) | 4 (13.3) | .090 |
| Baseline PEF | 328.20 ± 130.67 | 320.00 ± 125.55 | .738 |
| Item | Ropivacaine (n = 25) | Placebo (n =30) | P Value |
|---|---|---|---|
| Puncture Zone | |||
| Intercostal | 2 (8.0) | 2 (6.7) | .789 |
| Subcostal | 23 (92.0) | 28 (93.3) | .789 |
| Number of Punctures | |||
| One | 24 (96.0) | 29 (96.7) | .859 |
| Two | 1 (4.0) | 1 (3.3) | .859 |
| Duration of Surgery, h | 2.51 ± 0.90 | 2.31 ± 0.74 | .187 |
| Use of Analgesic at Recovery | 14 (56.0) | 13 (43.3) | .186 |
| Dose of Pethidine at Recovery, mg | 36.43 ± 5.91 | 36.15 ± 6.68 | .873 |
| Use of Analgesics (24-hour) | 20 (80.0) | 23 (79.3) | .929 |
| Dose of Pethidine (6-hour), mg | 36.88 ± 18.83 | 33.33 ± 13.34 | .358 |
| Use of Apotel | 2 (8.0) | 2 (6.7) | .789 |
| Residual Stone | 1 (4.0) | 2 (6.7) | .540 |
| Horseshoe Kidney | 1 (4.0) | 2 (6.7) | .540 |
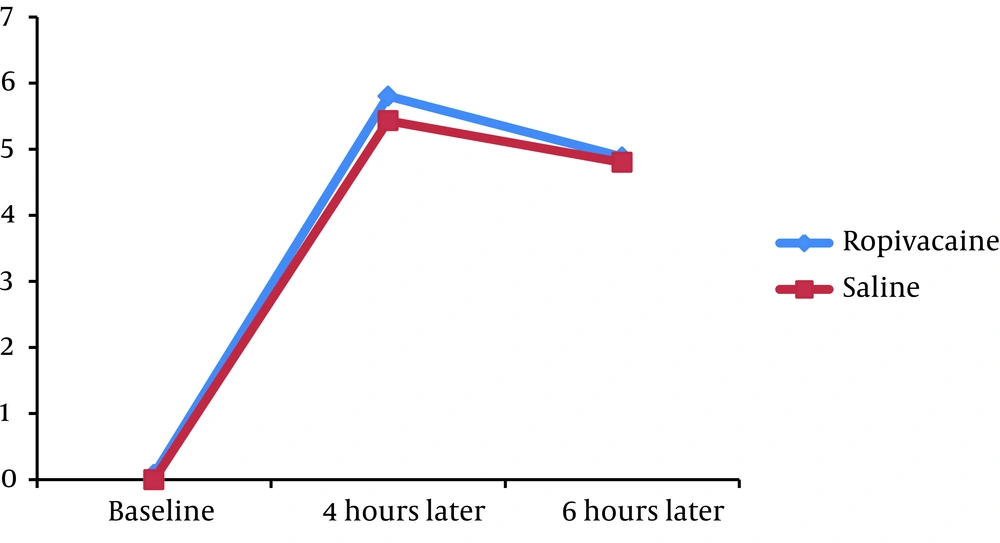
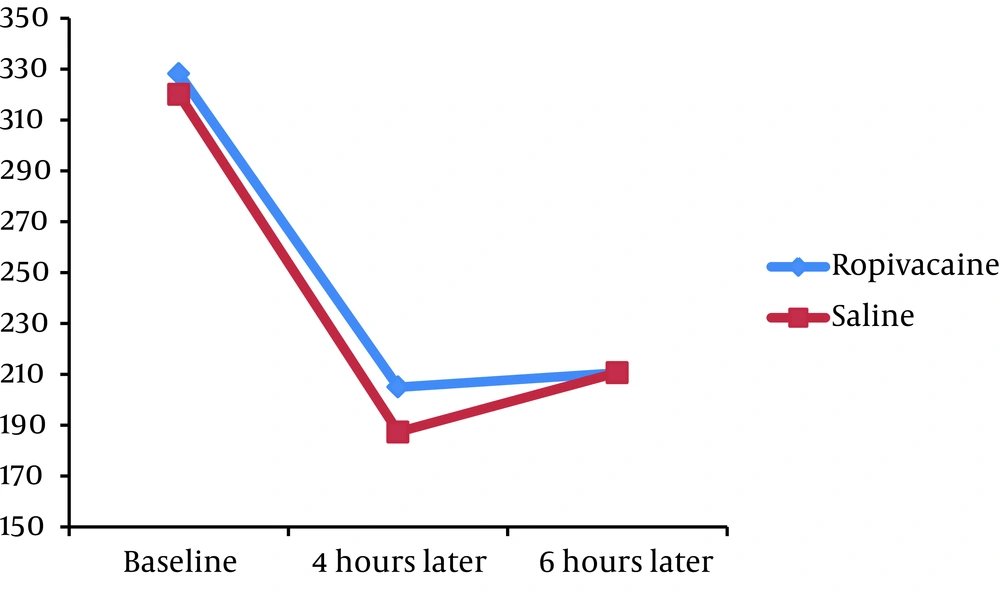
We found no significant difference in the mean pain severity score between the groups 4 hours (5.80 ± 2.12 versus 5.43 ± 1.76, P = 0.332) and 6 hours (4.88 ± 2.18 versus 4.80 ± 1.72, P = 0.830) after the operation. The mean PEF at baseline was similar in case and control groups (328.20 ± 130.67 versus 320.00 ± 125.55, P = 0.738). Moreover, no significant difference was revealed in PEF index 4 hours (205.00 ± 99.94 versus 187.33 ± 115.39, P = 0.398) and 6 hours (210.60 ± 105.85 versus 187.33 ± 115.39, P = 0.335) after PCNL.
Assessing the trend of changes in postoperative pain score showed that in the both groups, the mean pain severity score improved from 4 to 6 hours (P < 0.001 in the both groups) (Figure 1). Moreover, in the both groups, PEF index increased from 4th to 6th hour with a mild gradient after the operation (Figure 2).
5. Discussion
Based on most previous studies on postoperative pain, some local anesthetics have been demonstrated to be more effective than common and traditional medications such as bupivacaine and ropivacaine with more therapeutic effects and less postoperative analgesics need, but some studies had no impact on pain scores (16). The present study did not show the beneficial effects of ropivacaine on postoperative pain relief and PEF improvement. By administrating ropivacaine 0.2% (30 mL) via nephrostomy, patterns of changes in both pain severity and PEF were similar in both ropivacaine and placebo groups. The difference in pain severity from the fourth hour after the operation to 2 hours later was not significant between the groups.
Different results were found in some previous studies, while there were differences in the methods of these studies. In Parikh’s studies (6, 17-19) on patients undergoing elective PCNL, visual analogue scale and D-VAS in the bupivacaine group were significantly higher than the ropivacaine group during a few hours after the operation. In another study, the above mentioned criteria were higher in the placebo group than the ropivacaine. In another study, the above mentioned criteria were higher in the ropivacaine group than the ropivacaine and morphine groups. In these studies, in contrast to our study, nephrostomy tube was not withdrawn at the end of operation and postponed to a later time (on day 2). Moreover, in one of these studies, no comparison was made between a local anesthetic and a placebo (18). In one investigation, although the mean number of doses of tramadol and total consumption of tramadol in 24 hours were less in the ropivacaine group, the difference was not significant (19). In another study (6), VAS at rest as well as during deep breathing and coughing were significantly lower in ropivacaine group during the first 24 hours. The mean time of the first rescue analgesic in the ropivacaine group was also longer than the control group. The mean number of doses of tramadol in 24 hours in ropivacaine group was less than the placebo group. The mean total amount of tramadol in 24 hours in the ropivacaine was also lower than the placebo group. In Parikh’s studies, a spinal needle was inserted up to the renal capsule under ultrasonographic guidance along the nephrostomy tract at two positions and not at the main nephrostomy tube; so the probability of drug dilution by urine was less. The operation method was classic PCNL (which nephrostomy tube saves for several days) and patients having supracostal puncture and more than one puncture were excluded from the study. In a study by Gokten et al, there was no significant analgesic effect of levobupivacaine compared to the placebo; however, only levobupivacaine infiltration through the nephrostomy tract in combination with intravenous paracetamol infusion was safe and efficacious as an analgesia method (20). Moreover, they did not perform any measurements on the respiratory system, which might be affected by pain. They did not use any quantity criteria (only VAS which is a quality criterion in fact). In another study by Ugras et al. (4), at the end of the operation, 30 mL of either 0.2% ropivacaine or saline was instilled into the renal puncture site, nephrostomy tract and skin. VAS at 6 hours, time to first analgesic demand and total analgesic need were significantly lower in the ropivacaine group, whereas PEF at 2 and 6 hours were significantly higher. Analgesic use in the first 12 and 24 hours were lower in this group. Our study protocol was not similar to Ugras et al. (4) study, as in their study, nephrostomy tube was withdrawn on the second day (in contrast to our study, in which nephrostomy tube was withdrawn at the end of operation). The results of Ugras et al. (4) were different compared to our study and this may be due to the presence of differences, especially in the mentioned type of surgery. Urine leak into the injection site, Nephrostomy tract is a tract reaching the calyx. Urine flow appears in the nephrostomy tract before administrating the ropivacaine. The urine in the calyx is acidified. Acidified urine (in the calyx) may neutralize (chemical neutralization) the ropivacaine. Chemical neutralization effect the ropivacaine. Dilution and attenuation affect the ropivacaine too. This may be the most important reasons of ineffectiveness of local anesthesia injection. The glomerular filtrate of blood plasma is usually acidified by renal tubules and collecting ducts (21). The pH of medium containing local anesthetic affects drug activity by altering relative percentage of base and protonated forms. For example, in inflamed tissues, the pH is lower than normal and local anesthetics are more protonated than normal tissue and consequently penetrate the tissue more slowly (22). The surface activities for uncharged anesthetics became higher than the charged ones (23). At lower pH than that corresponding to the pKa value of the local anesthetic, the amount of anesthetic adsorbed depended greatly to the membrane surface charge (24).
In conclusion, our study showed that instillation of ropivacaine 0.2% (30 mL) at whole surgical zones including renal puncture site (10 mL), nephrostomy tract (15 mL) and skin within tubeless PCNL surgery may not be effective on postoperative pain relief and improvement of PEF within 6 hours after the operation. Chemical evaluation of ropivacaine interaction and acidified urine in calyx is needed.