1. Background
Renal ischemia/reperfusion (I/R) injury, whether caused by shock, or during surgery or transplantation, remains a major cause of acute kidney injury (AKI). Reperfusion after ischemia initiates a complex and interrelated sequence of events that results in injury, causing mitochondrial swelling and loss of membrane potential and the eventual death of renal cells, as a result of a combination of both apoptosis and necrosis (1).
Apoptotic cell death has been documented in animal models and human biopsies after renal I/R (2), and inhibition of apoptotic signaling and cell death ameliorates the associated injury and inflammation in a murine model (3). Wen et al. demonstrated, in a study using the monoclonal antibody Ki-67, that tubular cell regeneration and proliferation were important elements of renal recovery in AKI (4).
Hyperbaric oxygen therapy (HBO) (5) is defined as a treatment in which a patient is intermittently exposed to 100% oxygen, while the treatment chamber is pressurized to a pressure above sea level (> 1 atmospheres absolute, ATA, 760 mmHg). HBO therapy has been used in a number of medical conditions with a proven efficacy for a limited number of disorders (6). However, the effects of HBO therapy on apoptosis and proliferative activity after I/R injury have not been fully understood.
2. Objectives
We studied the possible beneficial effects of HBO therapy on apoptosis and tubular cell regeneration after renal I/R injury in rats.
3. Materials and Methods
3.1. Animals
Animals were maintained on regular rodent chew and water ad libitum prior to the experiments. The experiments were conducted according to the guidelines of the institutional Animal Use and Care Committee.
3.2. Experimental Design
Male Sprague-Dawley (SD) rats (n = 30), weighing 250-280 g were randomized into three groups: Sham (Sham-operated rats); I/R (animals submitted to I/R); and I/R + HBO (I/R rats exposed to HBO).
Renal ischemia was induced by unilateral occluding of the left renal artery for 30 minutes with non-traumatic vascular clamps after right nephrectomy in rats. HBO therapy was delivered using an hyperbaric chamber (Model P-5100, Hanyuda Iron Works, Tokyo, Japan) at 2.5 atmospheres absolute (ATA). Animals underwent one session of 60 minutes each at one hour after initiation of ischemia. Sham-operated rats received similar operative procedures, however, the renal artery, after isolation, was not occluded and the incision was closed; these were defined as the control group.
At the end of the study, the rats were sacrificed under anesthesia with sodium pentobarbital (50 mg/kg intraperitoneally) at 24 hours and 48 hours following initiation of ischemia. The left kidney was harvested for analysis, since the apoptotic positive cells and proliferative reaction after I/R injury were well expressed at 24 and 48 hours, respectively.
3.3. Animal Care
These animal experiments were in compliance with the law and management of animal welfare in Japan (No. 105, 1973 Law) and the national institutes of health. Animals were given standard rat chow, and the animals had access to water ad libitum. They were housed in cages with controlled temperature (24-26°C) and with a 12 hour light/dark cycle. Rats used in the experiment were euthanized by deep anesthesia.
3.4. Renal Histology
The kidney were perfused in site, excised and prepared as two mid-coronal sections for embedding in paraffin. Sections were stained with hematoxylin-eosin and analyzed.
3.5. Immunochemistry Analysis
3.5.1. TUNEL Positive Nucleus
Paraffin-embedded renal biopsy specimens were processed for immunochemistry. We examined apoptotic cells by TdT-mediated dUTP nick end labeling (TUNEL method). The TUNEL assay was carried out according to the method of Gavrieli et al. with a slight modification as described previously. Paraffin-embedded tissues were cut into 5 μm thick sections and placed onto silane-coated slide glasses. Then, sections were deparaffinized with toluene and rehydrated in serially-graded ethanol solutions. After washing with PBS, the sections were treated with 10 μg/mL proteinase K in PBS for 15 minutes at 37°C. The sections were then incubated with 1 × TdT buffer (25 mM Tris-HCl buffer, pH 6.6, containing 0.2 M potassium cacodylate and 0.25 mg/mL BSA) alone for 30 minutes at room temperature. After incubation, the slides were reacted with 800 units/mL TdT dissolved in TdT buffer, supplemented with 1.5 mM CoCl2, 0.5 μM biotin-16-dUTP, 20 μM dATP, and 0.1 mM dithiothreitol for 90 minutes at 37°C. The reaction was terminated by washing with 50 mM Tris-HCl buffer (pH = 7.4), and endogenous peroxidase activity was inhibited by immersing the slides in 0.3% H2O2 in methanol for 15 minutes at room temperature followed by washing with PBS. After incubation with 500 μg/mL normal goat IgG in 5% BSA in PBS for 60 minutes at room temperature, the sections were incubated with HRP goat anti-biotin antibody (1: 100) diluted with 5% BSA in PBS for 60 minutes at room temperature. After washing with 0.075 Brij L23 in PBS, the HRP sites were visualized with DAB and H2O2 in the presence of cobalt and nickel ions. As a negative control, adjacent sections were subjected to reaction without TdT in every experiment (7-10).
3.5.2. Ki-67 Positive Cells
Cellular proliferation activity was determined with an antibody using anti-Ki-67 antibody (NB600-1252, Novus Biologicals, CO, USA). Ki-67 is one of the nuclear proteins related to the cell cycle. It is expressed in the G1-phase, S-phase, G2-phase, and M-phase, as seem by the existence of proliferating cells, and is not expressed in the G0-phase, when the cell stops proliferating. Samples were blocked by the solution (1% bovine serum albumin and PBSTN containing 0.5% gelatin), and secondary antibody (Alexa Fluor 488 anti-Rabbit) was added and stained after washing thoroughly with PBS, after an overnight incubation at 4°C. Cell nuclei were counterstained and sealed with DAPI (Dojindo Laboratories Co., Ltd., Japan) (11).
Immunoreactive stained areas were observed using an Olympus BX51 brightfield microscope (Olympus, Tokyo, Japan) with attached CCD camera. Apoptotic positive cells and positive cellular activity against anti-Ki-67 antibody were observed and determined in the area of the cortex and the outer stripe of the medulla (OSOM) under magnification ×20 and ×200, respectively. For each section, 10 microscopic fields were randomly examined. Quantitative histomorphometric analysis was determined by means of image analysis software (ImageJ, U. S. National Institutes of Health, Bethesda, Maryland, USA).
3.6. Statistical Analysis
The results are expressed as Mean ± SD. Comparisons between the three groups with the software StatMate IV (ATMS Co., Ltd., Tokyo, Japan) was performed using one-way analysis of variance (ANOVA) and Tukey-Kramer test. A P < 0.05 was defined as a significant difference.
4. Results
4.1. Histopathological Findings
The I/R group demonstrated necrosis characterized by interstitial swelling and epithelial cell shedding and hollowing in the proximal and distal tubules of the cortex and the medulla. However, the I/R + HBO group partially attenuated the histological changes after renal I/R.
4.2. Immunochemistry Findings
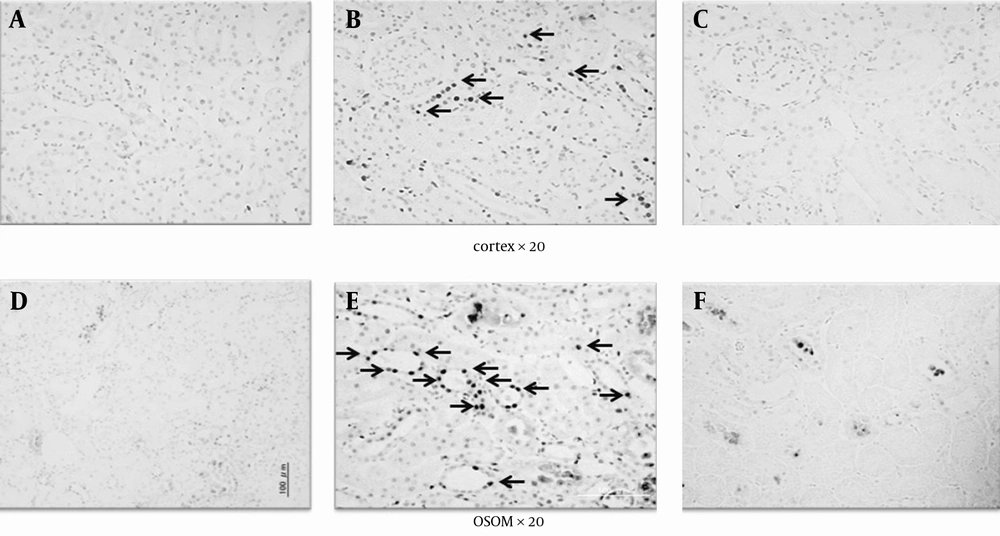
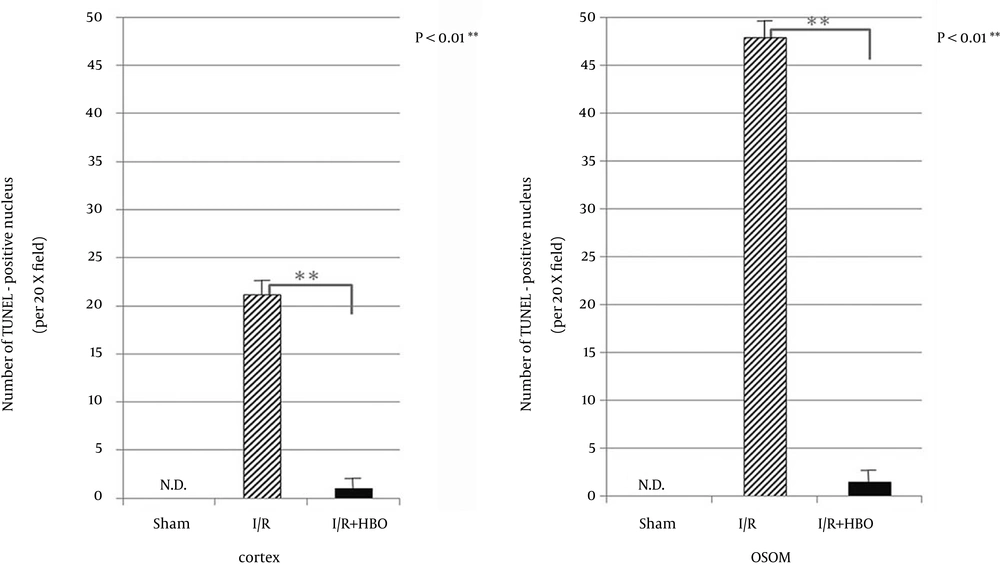
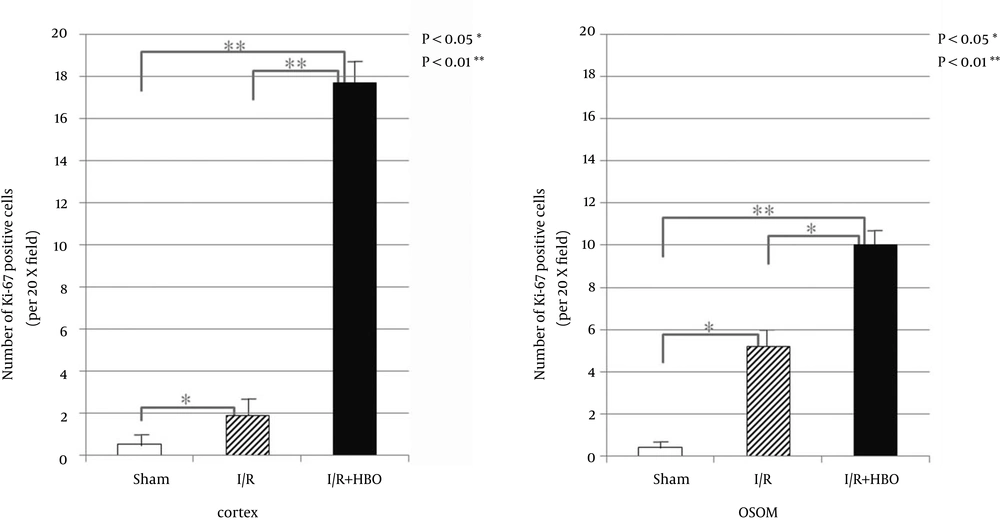
Apoptosis characterized by TUNEL positive staining was frequently observed in the distal tubule of the cortex and the outer stripe of the medulla (OSOM). On the other hand, a remarkable decrease in TUNEL positive cells was observed in the I/R + HBO group (Figure 1). Figure 2 summarizes the immunohistological data of TUNEL positive nucleus in the cortex and OSOM of the Sham-operated and I/R groups with or without HBO therapy. There was a marked increase in the number of TUNEL positive nuclei in the I/R group compared to the Sham group. However, HBO therapy significantly attenuated the effects of I/R injury on the appearance of apoptotic nucleus in both the cortex and the OSOM. Figure 3 summarizes the results of immunostaining for anti-Ki-67 antibody positive cells, a marker for proliferative cell reaction, in the cortex and the OSOM. HBO therapy significantly increased the proliferative reaction after renal I/R compared with the Sham and I/R groups. Ki-67 positive cells were observed in the cortex (1.9 ± 2.1, 17.7 ± 3.9/field) and the outer stripe of the medulla (5.2 ± 3.7, 10 ± 4.3/field) in the I/R and I/R + HBO groups, respectively. A significant increase in Ki-67 positive cells was found in the I/R + HBO group in both the cortex and the OSOM (Figure 3).
Sections in the cortex and the outer stripe of the medulla (OSOM) are shown at the top and bottom, respectively. A and D, issue sections obtained 24 hours after sham surgery in the cortex and OSOM, respectively.; B, E, C, F, The I/R group showed a remarkable increase in apoptotic positive nucleus (arrow in B and E) in both the cortex and the OSOM, compared to the Sham and I/R + HBO groups (C and F).
TUNEL-positive nucleus number in the cortex (21.2 ± 8.5/field) and the outer stripe of the medulla (OSOM) (47.9 ± 18.0/field) were observed in the I/R group. The I/R + HBO group showed the number in the cortex (1 ± 0.1/field), OSOM (1.5 ± 0.2/field). A significant reduction in positive cells was observed in the I/R + HBO group (P < 0.01). No TUNEL positive nuclei were not found in the Sham group. ND: not detected.
5. Discussion
In this study, we demonstrated that early hyperbaric oxygen (HBO) therapy suppressed apoptosis with proliferative reaction after renal ischemic reperfusion injury (I/R) in rats. Renal I/R is a common factor in the development of acute kidney injury (AKI), which has been recognized as resulting in high morbidity and mortality. Reperfusion is vital for the ischemic renal tissue but has the potential to aggravate tissue injury (12). Several studies have shown the beneficial effects of HBO on the histopathological changes after renal I/R (13, 14). Cellular death that occurs after I/R injury was morphologically divided into apoptosis and necrosis, both of which are positioned as different types of cellular death (15, 16). There were no reports about the effects of HBO on apoptosis and repaired pathway after renal I/R, to the best of our knowledge.
The apoptotic cells showed the various morphological changes as chromatin aggregation, fragmentation of DNA, and cell membrane blebs (projections) in the early stage and finally, disappearance by phagocytosis. Meanwhile, necrosis develops as degradation of the nucleus, the cytoplasm and lysosomal membrane in the cells and causes an inflammatory response. Schumer et al. (16) described morphologically that sustained necrosis developed just by renal ischemic injury, and reperfusion after renal ischemia significantly induced apoptosis.
By continuing the death of two types of cells, renal failure is sustained and leads to poor prognosis. In the caspase cascade, the initiator and effector are stimulated to cause activation of the cascade and induce irreversible apoptosis (17, 18). That the distal tubule is easily injured by I/R is consistent with a previous study by Kreisberg et al. (19) however, the exact mechanism might not be fully explained at the moment.
Apoptotic cells, 24 hours after I/R injury, were significantly observed by the TUNEL method in the distal tubule of the cortex and the medulla in the I/R groups, but were not observed in the I/R + HBO group, suggesting that HBO treatment inhibited the activity of caspases.
The following studies support our findings regarding inflammation, apoptosis and proliferation after the injury.
Sharples et al. reported, biochemically and histologically, that erythropoietin prevented caspase-3,-8 and -9 activation in vivo and reduced apoptotic cell death caused by renal I/R in rats (1).
Wang et al. showed that netrin-1, a diffusible laminin-related protein, protected renal tubular epithelial cells against renal I/R by suppressing apoptosis and increasing proliferation in transgenic mice (20). In addition, suppression of apoptosis induced by renal I/R prevented inflammation using a murine model (2).
Wen et al. described tubular cell regeneration and proliferation as an important element of renal recovery in acute renal injury. The mechanisms involved in the protection by HBO against renal I/R remain unclear. However, HBO therapy would suppress apoptosis through the caspase cascade after I/R injury in the ischemic region (4).
Although biochemical analysis was not simultaneously determined in this study, we previously examined the values of serum creatinine (Cr) and blood urea nitrogen (BUN) in the same fashion, with or without HBO therapy in rat models (not published). Significant decreases in Cr (3.8 ± 0.2 vs. 2.7 ± 0.1 mg/dL) and BUN (120 ± 2.2 vs. 105 ± 2.5 mg/dL) were found 24 hours after renal I/R injury between the I/R and I/R + HBO groups, respectively. These results were matched with another study of Ramalho et al. (14) describing the significantly lower levels of Cr and BUN, suggesting that HBO therapy ameliorated renal function after the injury.
This study has some limitations because the number of animals was too small to understand fully the profiles of the histological benefit of HBO after the injury. Further studies will be necessary to elucidate the precise mechanism of HBO therapy and repaired pathway, including apoptosis after renal I/R injury.
In conclusion, in this study, we have demonstrated that hyperbaric oxygen (HBO) therapy attenuated the expression of apoptosis induced by renal I/R injury and promoted renal tubular regeneration.