1. Background
Academic bodies in Japan that are concerned with hemodialysis have proposed standards for hemodialysis water quality management. These standards have undergone many changes, and there are ongoing efforts to ascertain and improve the current state of dialysate purification (1, 2). In 2011, the international organization for standardization (ISO) issued its own guidelines on dialysate purification (3). However, in addition to management of dialysis water quality, control of dialysis piping is also extremely important in dialysate purification.
It is important to keep dialysate line piping as simple as possible. Dead space and unnecessary bends, joints, branches, and steps (differences in elevation) should be kept to the necessary minimum, and care must be taken to prevent the adhesion of contamination sources on the inner walls of the piping.
Dialysis piping is cleaned in order to remove any bacterial growth or materials deposited inside the piping from discharged dialysate. The piping for the collection of dialysate and discharge is at high risk of bacterial growth and must be chemically disinfected using a cleaning agent. As bacterial contamination progresses when the flow is slow or has stopped in the dialysis piping, loading the piping with disinfectant at these times is useful in suppressing bacterial growth and preventing the formation of contamination sources, such as biofilms (4, 5).
Biofilm is an aggregate of microorganisms enclosed within a self-produced slime, with extracellular polysaccharides (EPS) playing a major role in biofilm formation. Biofilms can form anywhere in a water environment, and the majority of contaminating bacteria are believed to live in biofilms. Some of these bacteria are beneficial, but problems arise because biofilms typically support bacteria that corrode piping or contaminate water. Biofilms have also been linked to infection via medical devices, such as catheters and pacemakers, as well as hemodialysis equipment (6-8). As bacteria may be present anywhere inside the dialysis piping, it is essential that the cleaning disinfectant reaches everywhere within it. For effective disinfection, the entire piping system must be completely disinfected, including the end region, where the disinfectant concentration tends to become diluted. Once formed, biofilms tend to be resistant to the effects of cleaning disinfectants and become difficult to remove.
In Japan, cleaning disinfectants have been clinically evaluated based on endotoxin levels and bacterial counts, but there are no published studies evaluating the biofilm removal efficacy of these agents at the electron microscope level.
2. Objectives
In this study, we used electron microscopy to evaluate the efficacy of various cleaning disinfectants at removing biofilms from hemodialysis piping.
3. Methods
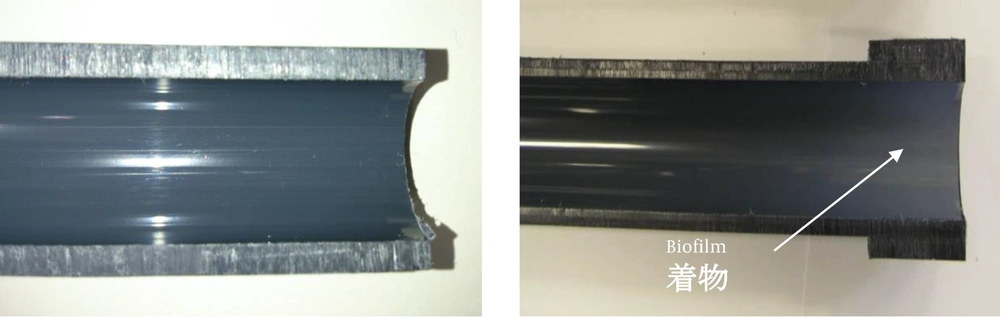
We evaluated rigid polyvinyl chloride (PVC) dialysis piping that had been used for two years at a dialysis center. We also evaluated ten dialysis instruments at five dialysis centers. The inner surface of the sections of piping for evaluation contained biofilms with white adhesions that were visible to the naked eye (Figure 1). The pipe was severed by snapping after immersion in liquid nitrogen. An unused rigid PVC pipe was used for comparison.
Three different cleaning disinfectants were used: 12% sodium hypochlorite (Asahi Kasei Advance Co., Ltd., Tokyo, Japan), 99% acetic acid (Wako Pure Chemical Industries, Ltd., Osaka, Japan) and 1,000 ppm Steracare peracetic acid-based bactericidal detergent (Asahi Kasei Medical Co., Ltd., Tokyo, Japan).
These disinfectants were tested at room temperature and heated (80°C). For cleaning at room temperature, the pipe section was immersed in the cleaning disinfectant for 2 hours, after which the effectiveness of biofilm removal from the pipe surface was evaluated using a scanning electron microscope (SEM). For hot cleaning, the pipe section was submerged in the cleaning disinfectant and cleaned at 80°C for 2 hours, using a heated stirrer (SW-600H;Nissinrika, Ltd., Tokyo, Japan), after which the effectiveness of biofilm removal from the pipe surface was evaluated with SEM (JSM 6510LV; JEOL Ltd., Tokyo, Japan).
4. Results
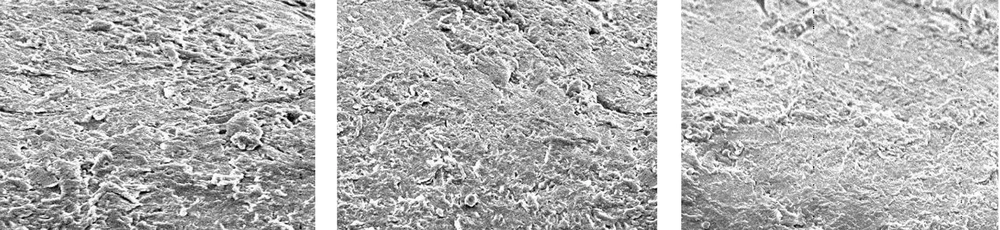
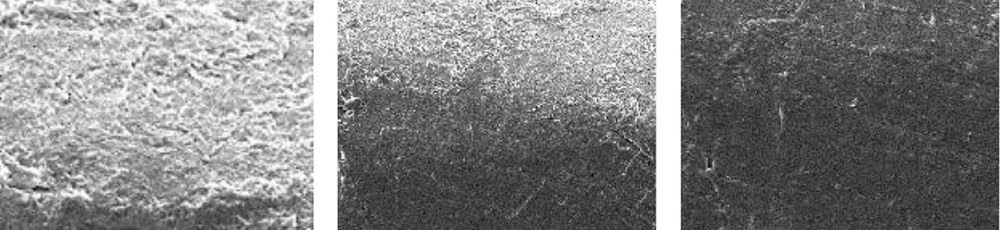
Figure 2 shows an SEM image of the unused pipe with no adhesions. Figure 3 shows the results with sodium hypochlorite after room-temperature and hot cleaning, compared to before cleaning. Room-temperature and hot cleaning both removed some surface biofilm, but removal was poor when compared to the uncleaned pipe.
Figure 4 shows the results for acetic acid after room-temperature cleaning and hot cleaning, compared to before cleaning. Acetic acid was more effective than sodium hypochlorite at removing the surface biofilm at room temperature, compared to the uncleaned pipe. Hot cleaning with acetic acid was even more effective at biofilm removal than room-temperature cleaning. However, although hot cleaning with acetic acid was more effective at removing biofilm than hot cleaning with sodium hypochlorite, it was still insufficiently effective at removing the deeper layers of biofilm.
Figure 5 shows the results for peracetic acid (Steracare) after room-temperature cleaning and hot cleaning, compared to before cleaning. Peracetic acid was more effective than both sodium hypochlorite and acetic acid at removing the surface biofilm at room temperature, when compared to the uncleaned pipe. Hot cleaning with peracetic acid was even more effective at biofilm removal than hot cleaning with sodium hypochlorite and acetic acid, and was also highly effective at removing the deeper layers of biofilm.
5. Discussion
Various cleaning disinfectants are currently used in Japan for the management of dialysis piping. Dialysis centers also differ in type, method, and frequency of use of cleaning agents, rather than following a standardized approach. For this study, we used chemical agents at much higher concentrations than would normally be used in clinical practice, and investigated their biofilm removal efficacy under extreme conditions.
Sodium hypochlorite is a low-cost agent with a wide antibacterial spectrum, but it was not effective at removing biofilms at normal or high temperatures. Cappelli et al. (9) attributes this ineffectiveness to the bacteria being protected by a viscous liquid formed inside the pipe as a result of the oxidation of proteins on the biofilm surface by the disinfectant. Cleaning with sodium hypochlorite was able to remove a small amount of surface biofilm, but the effects were rather weak.
Cleaning with acetic acid removed a certain amount of surface biofilm at room temperature and a greater amount when hot, but could not completely remove the biofilm down to the deeper layers. As the solubility of carbonate in acetic acid is high, the calcium carbonate that adheres to the biofilm surface is removed by cleaning with acetic acid; however, this agent was clearly unable to remove the deeper layers of biofilm.
Cleaning with peracetic acid had a strong biofilm removal effect at both room temperature and when heated. This is because peracetic acid is strongly bactericidal; its constituent hydrogen peroxide removes the biofilm’s protein layer and its constituent acetic acid removes the carbonate. Heating also appears to further enhance the removal effects of this agent.
Biofilm formation begins with the adhesion of bacteria to a physical surface. Mucilaginous polymers are then formed, and finally a bacterial community is established. The factors thought to influence biofilm formation in pipes include the pipe’s material, surface area, surface structure, and flow rate. There is also evidence that the piping material has little or no effect on the growth of biofilms, and that bacteria are capable of adhering to all known piping materials (10). Surface area dimensions suited to the adhesion and growth of biofilms are found in the dialysis system environment. Surface structure also influences the speed of contamination, which is slower on smooth surfaces than rough surfaces. A faster flow rate can inhibit biofilm growth, but is thought to be unable to prevent bacterial adhesion to pipe surfaces (11). It is reasonable to assume that the use of unsuitable cleaning disinfectants could lead to the remaining biofilm forming an even stronger barrier, or could allow biofilms to form inside the piping in a short time.
Water quality management based on the determination of endotoxins and bacterial counts has been prioritized in dialysate purification. Although this is useful in making judgements about microbial contamination in the dialysate production process and the outcome of cleaning disinfections, these results have little meaning unless biofilm countermeasures are also taken into consideration. Also, a number of studies have reported the clinical efficacy obtained by improving the cleanliness of the dialysate, (12, 13) and it is believed that maximum effort should be made to improve the cleanliness of dialysates.
5.1. Conclusion
Cleaning and disinfection using disinfectants at a high temperature and high concentration effectively removes biofilms from hemodialysis piping. However, long-term exposure to cleaning disinfectants may affect the piping material. Established biofilms show resistance to cleaning disinfectants and could become a constant cause of biological contamination. The suppression of biofilm formation in dialysis piping is an essential part of dialysate purification strategies.