1. Introduction
Renal cell carcinoma (RCC) is unique among urologic tumors because of its potential for tumor thrombus formation and migration into the venous system (1). This develops in approximately 4% - 10% of cases (2) and some (2% - 10%) may extend up to the right atrium (3). RCCs are chemo- and radio-resistant, and due to the impending catastrophic nature of these tumors and also to recent advances in targeted therapy, an aggressive surgical approach has been advocated (4). Managing these situations is not only a surgical challenge but also has prognostic implications, (5) with an overall 5-year survival rate of 30 to 60% (6).
Extensive tumor thrombi can be approached by either thoracoabdominal or abdominal (with midsternotomy) incisions. Both approaches will provide excellent exposure to the diseased kidney, inferior vena cava (IVC), liver, and heart, and will facilitate the use of cardiopulmonary bypass (CPB) (2, 7). CPB with or without deep hypothermic circulatory arrest (DHCA) is recommended by many groups for high-level IVC tumor thrombectomy; however, some opposing views have advocated its use only for level IV thrombi (2, 7, 8). Most importantly, CPB requires expensive and sophisticated equipment, as well as highly trained and experienced staff. In addition, CPB with or without DHCA can lead to hematologic (platelet dysfunction and coagulopathy) and neurologic complications. Also, extensive heparinization in CPB could result in uncontrollable bleeding from raw surgical surfaces (9, 10). Hence, avoiding CPB while not compromising the aims of the cancer surgery or the patient’s prognosis is a logical approach that should be pursued. This is especially suitable for low-income communities. Different surgical methods for radical nephrectomy with IVC tumor thrombectomy without CPB have been previously reported. However, to the best of our knowledge, we report for the first time intrapericardial control without CPB for radical nephrectomy with IVC tumor thrombectomy.
2. Case Presentation
Between March 2008 and November 2015, of the 32 patients in our hospital with RCC and IVC tumor thrombi, six patients with supradiaphragmatic IVC tumor thrombi underwent radical nephrectomy with thrombectomy using the intrapericardial control technique. Our department is an educational tertiary uro-oncology center with a uro-oncology fellowship program. The demographic and clinical characteristics of these six patients are summarized in Table 1. All of the patients’ data, including pre- and intraoperative findings, postoperative complications, pathology reports, and follow-up information, were prospectively recorded. The tumor characteristics and thrombus level were preoperatively evaluated by computed tomography (CT) scanning and magnetic resonance imaging (MRI). The thrombus level was double-checked with transesophageal echocardiography (TEE) during surgery. None of the patients had imaging evidence of IVC wall invasion or metastases, and the thrombi were Mayo level IV (11) in all cases, with no intra-atrial components. Each patient’s performance status was determined according to the Eastern cooperative oncology group (ECOG) criteria. The cardiac, pulmonary, and renal functions of all patients were evaluated prior to surgery. The operation time was determined from anesthesia induction to completion of the skin closure. Postoperative follow-up evaluations included physical examinations and blood chemistry testing at one, three, and six months, then every six months for the next two years and yearly thereafter. Beginning three months postoperatively, abdominopelvic CT scans and chest X-rays were performed at every visit as follow-up imaging modalities.
| N | Sex | Age | Side | ECOG | Cardiac Risk | Tumor Size, cm | Histology | Fuhrman grade | pTNM |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 66 | R | 1 | High | 12 × 10 | Clear-cell RCC | 4 | T3cNxMx |
| 2 | F | 46 | R | 2 | Moderate | 15 × 10 | Papillary RCC type 2 | 3 | T4NxMx |
| 3 | F | 57 | R | 1 | Moderate to high | 11 × 10 | Clear-cell RCC | 2 | T3cNxMx |
| 4 | F | 62 | R | 0 | Moderate | 7 × 6 | Clear-cell RCC | 2 | T3cNxMx |
| 5 | F | 62 | R | 1 | Moderate to high | 9 × 6 | Papillary RCC type 2 | 3 | T3cNxMx |
| 6 | M | 75 | L | 2 | Moderate to high | 8 × 7 | Clear-cell RCC with sarcomatoid differentiation (5%) | 4 | T4NxMx |
Abbreviations: RCC, renal cell carcinoma; M, male; F, female; R, right; L, left; T, tumor; N, lymph node; M, metastasis; ECOG, Eastern cooperative oncology group performance status.
2.1. Surgical Technique
The positions of the thrombi were determined immediately after induction of anesthesia by TEE. A midline laparotomy with a midsternotomy incision was made in all patients. This technique was chosen because it allows a single incision to provide good access to the entire IVC, renal pedicle, and contralateral kidney, and it is a convenient approach for a probable cardiopulmonary bypass or an emergent pulmonary emboli evacuation.
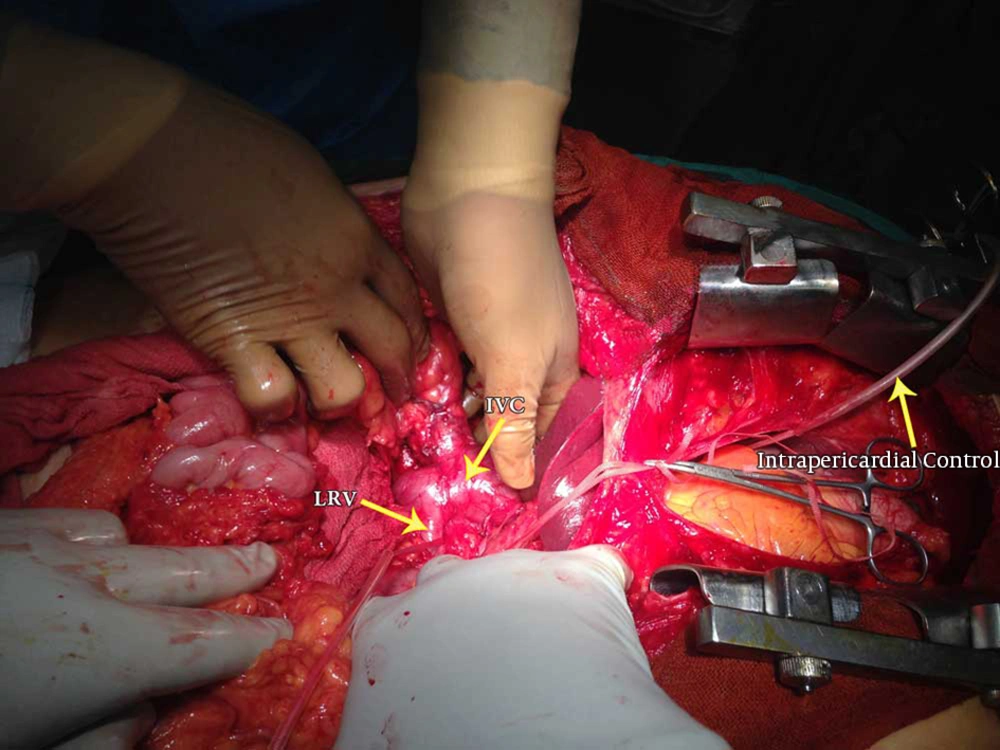
After confirmation of the infra-atrial position of the tumor thrombus by light touch, the intrapericardial IVC was isolated with a curved pedicle clamp and loosely controlled with a Rummel tourniquet. The surgery proceeded by exposing the infrarenal and infrahepatic portions of the IVC, as well as both renal veins and the ipsilateral renal artery (Figure 1). The renal artery was ligated as soon as possible to limit the amount of bleeding and to reduce cephalad extension of the tumor thrombus. Rummel tourniquets were loosely placed on the infrarenal part of the IVC and the contralateral renal vein, and the diseased kidney was fully mobilized with only the venous connection left intact. In one patient (number 3), because of a suspicion of major hepatic vein involvement, liver mobilization with the Pringle maneuver was also performed. After fastening all of the tourniquets, a longitudinal cavotomy incision from the renal vein insertion up to the short hepatic veins was made, and the kidney with the tumor thrombus was removed in its entirety by either digital manipulation or a tissue elevator. By establishing the retrograde flow from the infrahepatic IVC before closure, flushing of all tumor debris was ensured. In patient number 3, the cavotomy incision was extended until the major hepatic veins and tumor thrombus were extracted under direct visualization. In patient number 2, because of infrahepatic caval wall invasion, that segment was excised and the distal IVC was anastomosed to the left renal vein. After complete resection, the cavotomy was closed with double running sutures (5-0 Prolene). Removal of intrapericardial tourniquets was performed under TEE control to check for the occurrence of any tumor emboli. After meticulous hemostasis, a drain was placed in the pericardial space and the sternum was closed. Another drain was placed within the renal bed, and the laparotomy incision was closed in layers.
The total mean duration of surgery was 315 minutes. The mean amount of transfused blood was 4.33 units during surgery and 0.8 units in the postoperative period. Two patients did not require any perioperative transfusions. All patients were monitored in the surgical intensive care unit (SICU) for an average of 2.83 days (range 1 - 5). The average duration of hospitalization was 8 days (range 5 - 17). Only one patient (number 6) required prolonged hospitalization, due to of due to fever and a collection at the surgical site; this patient was managed with percutaneous drainage and antibiotic therapy. The other complication was acute renal failure (ARF) in patient number 2, who was managed conservatively and discharged with a serum creatinine level of 1.8 mg/dL. Based on the Clavien-Dindo classification of surgical complications, these patients’ complications were grade 3 and 2, respectively. There were no immediate or 30-day postoperative deaths. None of the patients had thrombi fragmentations or pulmonary emboli based on intraoperative TEE findings and postsurgical symptoms. Table 2 shows the perioperative and postoperative data.
| N | Operation Time (min) | Intraoperative Packed Cell Transfusion (units) | Postoperative Packed Cell Transfusion (units) | ICU Stay (Days) | Total Hospital Stay (Days) | Postoperative Complications |
|---|---|---|---|---|---|---|
| 1 | 300 | 3 | 0 | 5 | 7 | None |
| 2 | 330 | 0 | 0 | 1 | 6 | Increased Cr level to 2.8 |
| 3 | 360 | 3 | 1 | 4 | 7 | None |
| 4 | 270 | 2 | 0 | 3 | 5 | None |
| 5 | 360 | 0 | 2 | 2 | 6 | None |
| 6 | 300 | 7 | 2 | 2 | 17 | Surgical site collection and fever |
Abbreviation: SICU, surgical intensive care unit.
Pathological examinations showed clear-cell RCC in four patients and papillary type 2 RCC in two, with additional sarcomatoid features in one of the specimens. The tumor stage was T3c in four patients and T4 in two because of ipsilateral adrenal involvement. The mean duration of follow-up was 33.5 months (range 3 - 94). Recurrence was detected in one patient (number 3) at the tumor bed 12 months after surgery, which was resected (clear-cell RCC, 7 × 6 × 3 cm in size) with no complications. She developed a second recurrence at the surgical incision site 27 months after the operation (clear-cell RCC, 4.5 × 3.5 × 3 cm in size) which was again resected. No evidence of metastases was evident with any of the recurrence episodes.
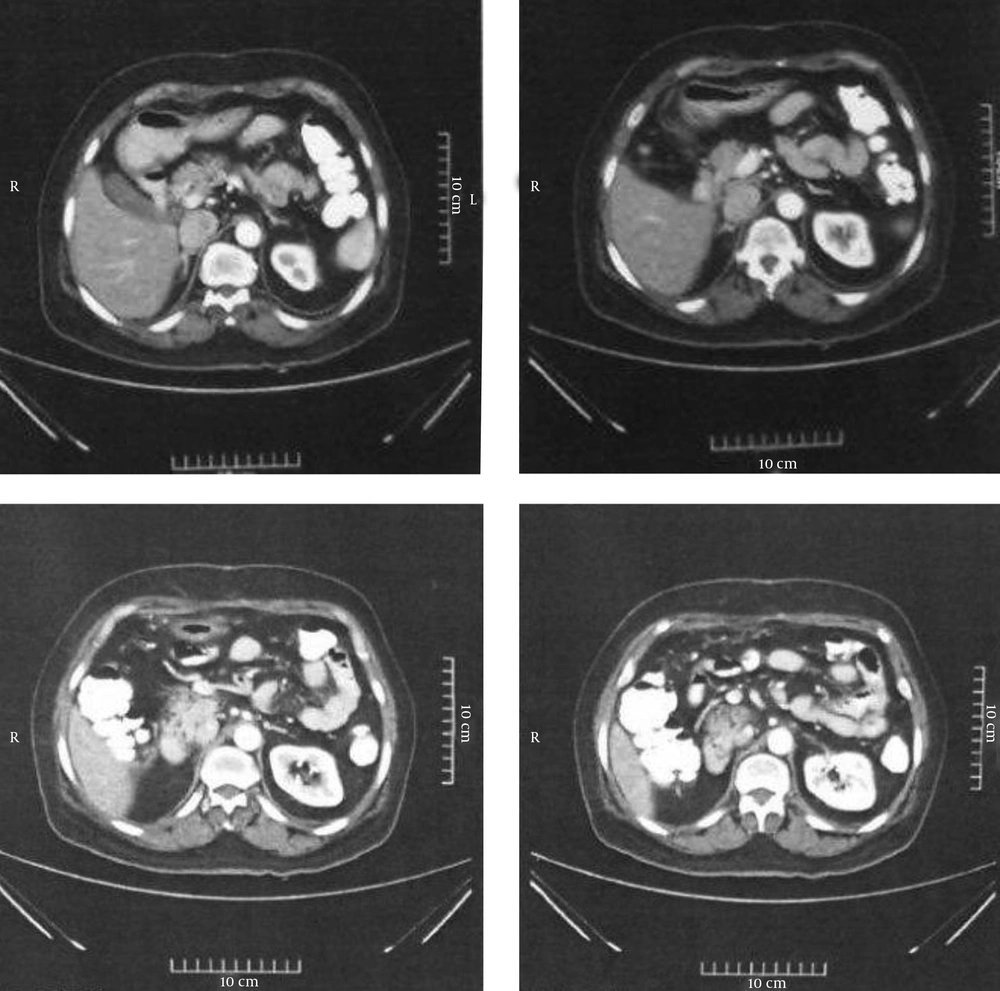
All of the patients are still alive at the time of this report. Figure 2 shows CT images for patient number 4 at the 18-month postoperative follow-up.
3. Discussion
RCC with vena caval extension is infrequent, but when it occurs, it necessitates an aggressive approach by a surgical team with extensive experience in urological cancer surgery (1, 12). The standard treatment for advanced tumor thrombi is radical nephrectomy and thrombectomy via laparotomy and midsternotomy incisions with atriotomy. This is possible only using CPB with or without DHCA (13, 14). CPB with hypothermia carries a known risk of perioperative bleeding due to platelet dysfunction and systemic heparinization (15). Other known risks of CPB are sepsis, multi-organ failure, and neurological deficits (15-17). In addition, specific and expensive equipment and highly trained personnel are required. Furthermore, the majority of hospitals lack the facilities for CPB, even in the wealthiest countries. Due to these complications and limitations, there are abundant recommendations to limit the use of CPB for only large intra-atrial thrombi, and to manage infra-atrial or small non-adherent atrial thrombi with non-bypass techniques (15).
There are several recommended non-CPB techniques in the literature with proven efficacy in terms of surgical perfection and overall survival. Ciancio and colleagues (15) described a technique used on 12 patients with supradiaphragmatic tumor thrombi. They performed the surgery through an abdominal incision using a transdiaphragmatic approach to the intrapericardial IVC, entering the right atrium. In their series, there were no complications, operative deaths, or pulmonary emboli related to the surgery. In another series by Patil and colleagues, 44 patients with Mayo level IV thrombi (35 supradiaphragmatic and nine intra-atrial) underwent radical nephrectomy and thrombectomy via right thoracoabdominal incisions and intra-pericardial control (4). No CPB or hypothermia was used. In their 34-year experience, Patil et al. had only one intraoperative mortality (in the supradiaphragmatic thrombus group), which was before 1990. Using this approach, they avoided complications related to vascular bypass techniques and achieved favorable survival outcomes at two and 10 years of follow-up, even in patients with nodal or distant metastases.
Sobczynski and colleagues (18) performed cavoatrial thrombectomies in four patients with an innovative Foley catheter-assisted technique, with no extracorporeal circulation or hypothermic arrest. All patients had level IV tumor thrombi with intra-atrial components. There was no intraoperative mortality or any recurrence after at least 12 months of follow-up. Their approach resulted in comparable or even better results with regard to blood loss and transfusion requirements compared to conventional or minimally invasive techniques. However, this technique is not suitable for large intra-atrial thrombi (i.e. those extending over 3 cm into the right atrium) or IVC wall infiltration.
Compatible with previous studies, our series also showed favorable results with regard to operation time, duration of hospital stay, and surgery-related complications. Our mean operation time was 315 minutes, which was lower than in the series by Ciancio et al. (15) and Patil et al. (4) (486 and 333.8 minutes, respectively). The mean number of units of blood required for transfusion during surgery in our study was 4.33, which is better than with other non-CPB techniques (4, 15). In Ciancio and colleagues’16 study of the intra-abdominal approach, a mean of nine units of red blood cells were required during surgery. This was 20.4 units in the study by Patil et al. (4) Because of our low number of patients, the complication rate cannot be compared with previous studies; however, we did not encounter any major morbidities or postoperative mortality in our patients. Postoperatively, the average length of stay in the ICU was 2.83 days (range 1 - 5), with a mean length of hospitalization of 8 days (range 5 - 17). In the studies by Patil et al. (4) and Ciancio et al.,(15) the mean hospital and SICU stays were 8.4 days (range 2 - 35) and 14.5 days (range 5 - 85), and 7 days (range 1 - 17) and 12 days (range 7 - 22), respectively.
3.1. Conclusion
Radical nephrectomy and IVC tumor thrombectomy by intrapericardial control without CPB or hypothermic circulatory arrest is a safe and effective procedure for level IV infra-atrial tumor thrombi. This procedure can avoid serious intra- and postoperative complications while providing acceptable cancer-control and mortality results. This technique is also applicable for small non-adherent intra-atrial tumor thrombi that are shrunk into the IVC after diseased renal artery ligation and can be milked downward before intrapericardial tourniquet placement.