1. Introduction
Retroperitoneal fibrosis (RPF) is a rare condition with an unclear etiology. It was first described in 1905, but more cases of this disease have since been reported (1, 2). RPF presents with the development of aberrant fibroinflammatory tissue in the retroperitoneal space, which can lead to entrapment and obstruction of the retroperitoneal structures, such as the ureters, aorta, and other abdominal organs (1, 3).
RPF can be primary or secondary. In primary RPF, the specific cause of disease is unknown, while in the secondary type, other factors, such as infection, neoplasm, trauma, surgery, radiotherapy, and certain drugs are the underlying factors that lead to the formation of RPF (2-4).
In this disease, systemic clinical manifestations are nonspecific, such as low-grade fever, fatigue, anorexia, weight loss, and myalgia (3, 5). It can also present with ureteral obstruction, lower extremity edema, varicocele, and renal failure (1, 2, 6).
In this case series, we present five patients with a history of opium addiction who presented with RPF, and describe their clinical manifestations, laboratory and radiologic findings, treatment, and follow-up.
2. Case Presentation
2.1. Patient 1
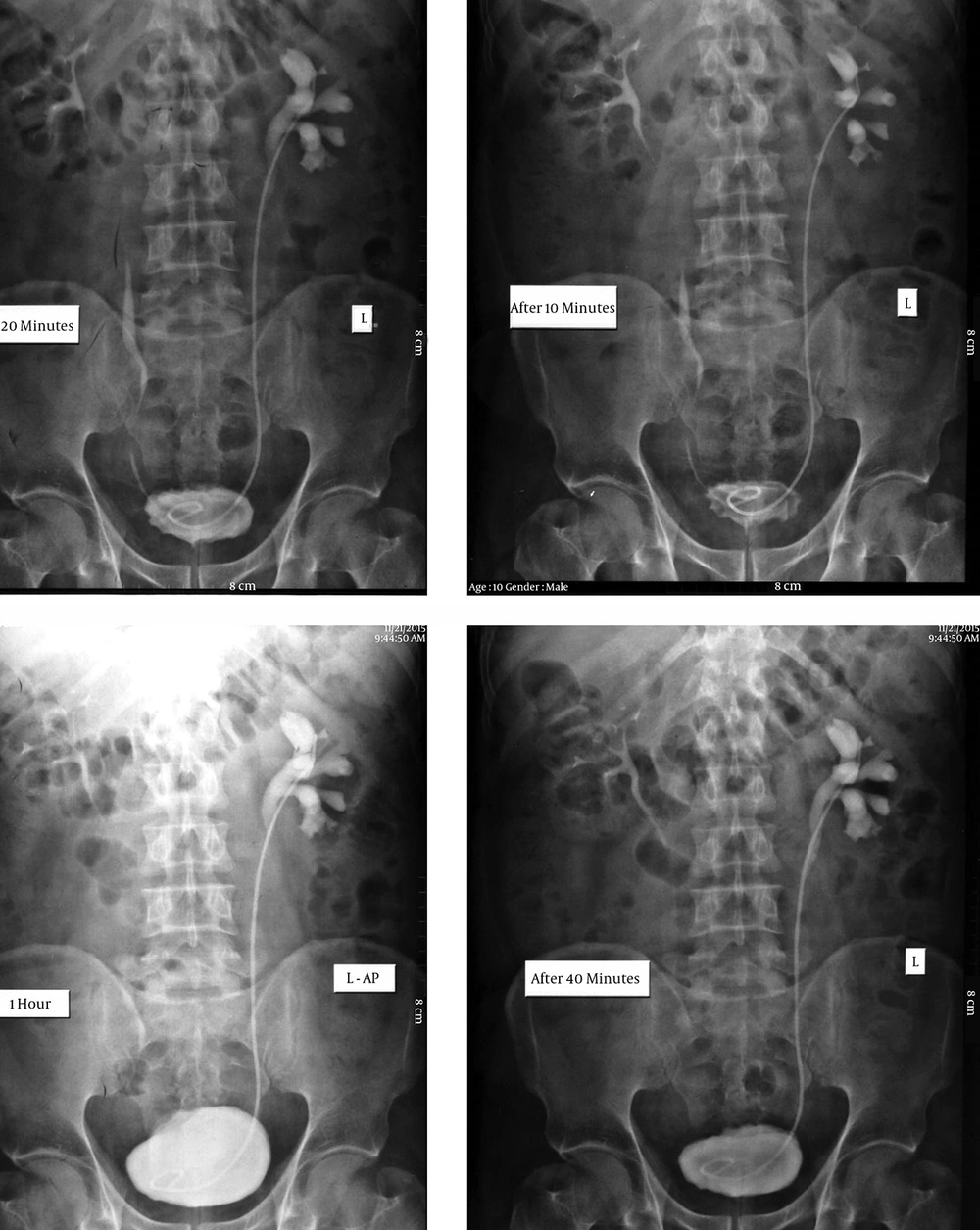
A 45-year-old man with a 3-year history of opium abuse presented with a 3-month history of left lower extremity edema. He had no previous history of any specific medical condition. Physical examination was normal, with a soft abdomen and normal bowel sounds. No pain was detected in the bilateral renal area. Laboratory tests revealed an elevated erythrocyte sedimentation rate (ESR) of 90 mm/h, and the complete blood count showed anemia with a hemoglobin of 11.2. Urine analysis was normal. Blood urea nitrogen (BUN) and serum creatinine were within normal range. Tumor markers, including CEA, CA125, αFP, and LDH were within normal limits. Ultrasonography detected unilateral hydronephrosis without ureteral dilation. Abdominal and pelvic computed tomography (CT) revealed an area of retroperitoneal thickness over the sacral promontory, with maximum thickness at the level of L4 - 5. Intravenous pylography (IVP) also revealed unilateral hydronephrosis (Figure 1).
The patient underwent double-J stent insertion to improve ureteral obstruction, and then a retroperitoneal biopsy was performed. The biopsy specimen was made up of collagenous tissue with inflammatory cells, compatible with RPF. The patient began taking an NSAID (ibuprofen 400 mg q 8 h) and prednisolone 1 mg/kg/day for approximately 6 weeks, and opium abuse was discontinued for a time. However, because the patient was refractory to medical therapy and there was deterioration of his clinical, laboratory, and sonographic findings, a surgical procedure was the best alternative therapy. Ureterolysis was performed immediately to improve renal function. Via a midline abdominal incision, the ureters were transpositioned to the intraperitoneal space and wrapped with omental fat to provide an effective barrier against re-entrapment by fibrotic tissue. After 6 months of follow-up, improvements in his clinical, radiologic, and laboratory findings were seen.
2.2. Patient 2
A 37-year-old male was admitted to the hospital with bilateral flank pain for 2 weeks. The patient had a 7-year history of opium abuse. He had no previous history of a specific medical condition. Physical examination was normal with a soft abdomen, but the exam was positive for bilateral costovertebral angle tenderness. Laboratory findings revealed elevated inflammatory markers (ESR = 70 mm/h and C-reactive protein [CRP] = 2+), mild anemia with a hemoglobin of 11.3, and elevated creatinine of 2 mg/dL. Urine analysis was normal and the results of the other biochemical screenings and electrolyte tests were all within normal limits.
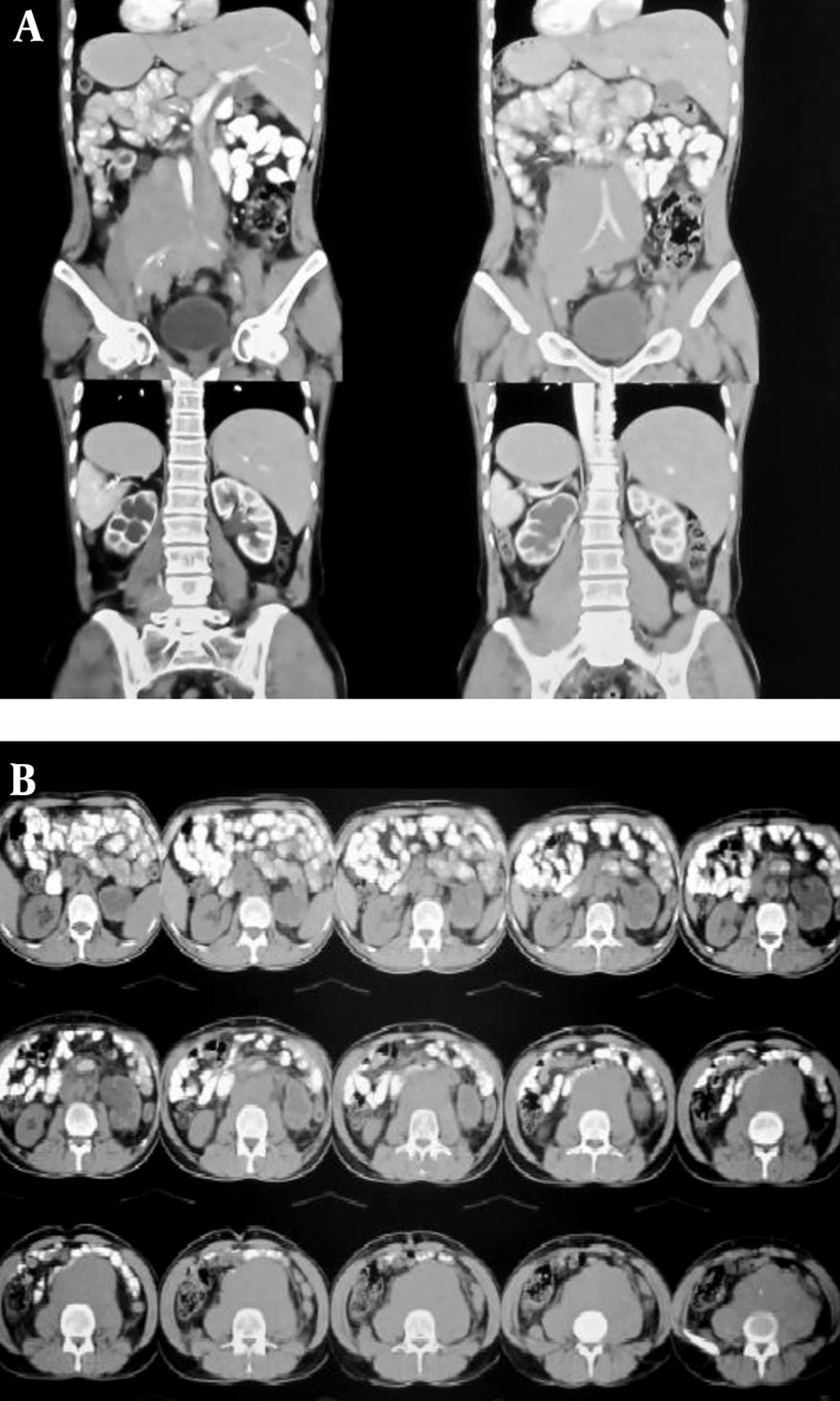
On ultrasonography, bilateral severe hydroureteronephrosis was detected. Urine drainage with a percutaneous nephrostomy tube (PCN) was done to improve renal function and creatinine levels. CT of the abdomen and pelvis was obtained, showing a retroperitoneal mass measuring 5 × 6 cm with compression effect on the lower aspect of the bilateral ureters, producing bilateral hydroureteronephrosis (Figure 2A, B). The definitive diagnosis was made with a tissue biopsy, which showed a collagen-rich background with fibroblastic elements lacking signs of atypia, with diffuse perivascular inflammatory cell infiltrations, compatible with RPF.
The patient underwent therapy with an NSAID (ibuprofen 400 mg q 8 h) and prednisolone (1 mg/kg/day) for approximately 4 weeks, and the opium was discontinued. The corticosteroid and NSAID were gradually tapered off within 6 months and the PCN tube was removed. Follow-up ultrasonography at 3 and 6 months showed a gradual remission of hydroureteronephrosis and at the last follow-up visit at 6 months, the ESR level was within normal range (< 10 mm/h).
2.3. Patient 3
A 48-year-old male with a 15-year history of opium addiction presented with a 5-month history of low back pain. Physical examination was normal, with no back or abdominal tenderness, organ enlargement, or palpable masses. Bilateral trace pedal edema was detected. Laboratory results showed mild anemia with a hemoglobin of 11 and an elevated ESR of 50 mm/h. BUN was 75 and serum creatinine was 3 mg/dL. Urine analysis was normal and the results of the other biochemical screenings and electrolyte tests were all within normal limits. Tumor markers, including CEA, CA125, αFP, and LDH, were also within normal limits.
Abdominal ultrasonography showed bilateral pyelocaliceal system dilation, and abdominal and pelvic CT revealed bilateral hydronephrosis and fibrotic tissue surrounding the abdominal aorta and common iliac arteries at the level of the sacral promontory. A double-J stent was inserted and retroperitoneal biopsy was performed. The biopsy specimen was made up of fibrotic tissue with inflammatory cells, compatible with retroperitoneal fibrosis. Medical treatment with an NSAID (ibuprofen 400 mg/dL) and prednisolone (1 mg/kg/day) was administered for 4 weeks. After clinical and radiologic improvement, the drugs were tapered off in 6 months and the PCN tube was removed. At the same time, the opium abuse was stopped. After 9 months of follow-up, the patient’s laboratory and radiologic findings were all within normal limits.
2.4. Patient 4
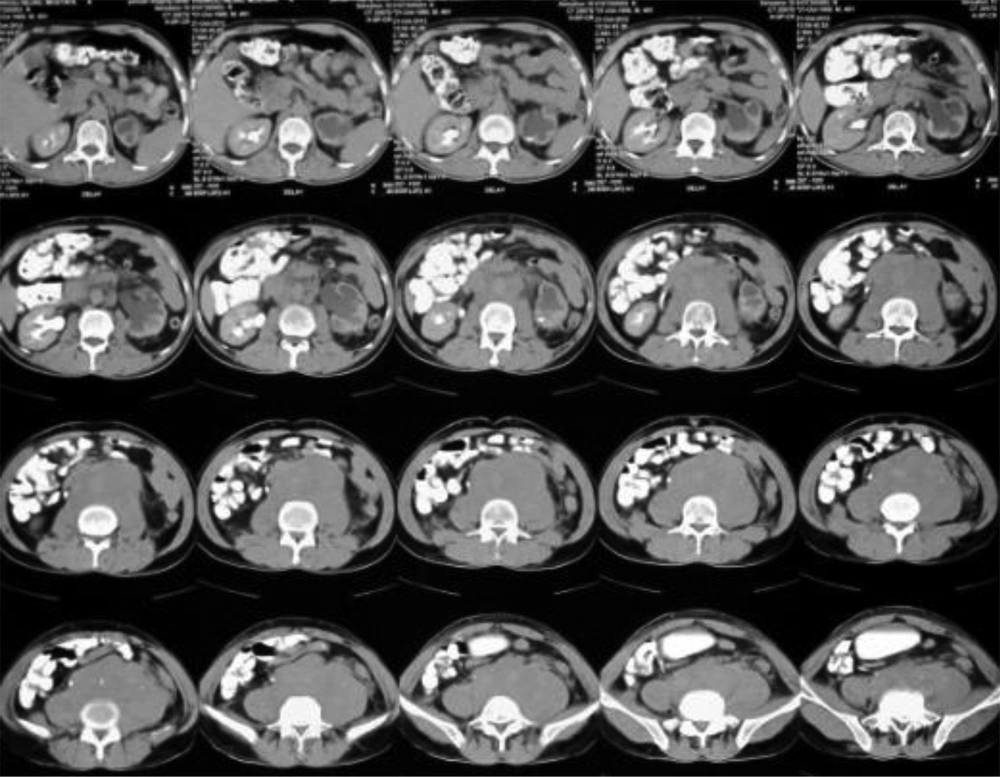
A 36-year-old man with a 10-year history of opium addiction presented with bilateral pedal edema and a 5-kg weight loss over the previous 9 months. He had no previous history of any specific medical condition. Physical examination was normal, with no back or abdominal tenderness. No costovertebral angle tenderness, organ enlargement, or palpable masses were identified. Laboratory tests revealed microscopic hematuria on urine analysis. BUN and serum creatinine were within normal range, and ESR was elevated (60 mm/h). The results of the other biochemical screenings and electrolyte tests were all within normal limits. Abdominal ultrasonography revealed unilateral pyelectasia, and abdominal and pelvic CT revealed a retroperitoneal mass (Figure 3). Post-void urine residue volume was normal.
After double-J stent insertion to correct ureteral obstruction, a retroperitoneal biopsy was performed. The result showed that the biopsy specimen was made up of fiber and fat tissue with infiltration of inflammatory cells, compatible with RPF.
The patient began taking an NSAID (ibuprofen 400 mg q 8 h) and prednisolone (1 mg/kg/day) for approximately one month, and was recommended to stop using opium. The NSAID and prednisolone were then tapered off at 8 months. On radiologic, laboratory, and clinical follow-up, full recovery was observed.
2.5. Patient 5
A 40-year-old female with a 5-year history of opium abuse presented with 6-month history of fatigue, low back pain, and menometrorrhagia. She had no previous history of any specific medical condition. Physical examination was normal and no abdominal tenderness, organ enlargement, or palpable masses were identified.
Laboratory tests revealed an elevated ESR (70 mm/h) and anemia with hemoglobin of 10.2. Urine analysis was normal. Serum creatinine was elevated (2.5). The tumor markers (CEA, CA125, LDH, αFP, and βHCG) were within normal limits. The results of the other biochemical screenings and electrolyte tests were all within normal range.
Ultrasonography revealed bilateral moderate hydronephrosis without ureteral dilation. On abdominal and pelvic CT, fibrotic tissue was observed surrounding the abdominal aorta and common iliac arteries at the level of the sacral promontory. A double-J stent was inserted and the patient began taking an NSAID (ibuprofen 400 mg q 8 h) and prednisolone (1 mg/kg/day) for approximately 6 weeks. She was also advised to stop using opium. After 9 months, the NSAID and prednisolone were tapered off, and improvements in the patient’s clinical manifestation and radiologic and laboratory findings were observed.
3. Discussion
RPF is a rare condition with a prevalence of 11.4/100,000 individuals and an incidence of 0.1/100,000 per year (2). RPF has an unclear etiology, and was first described by Albarron in 1905 (1, 2). However, more cases of this disease have since been reported (1, 7, 8). RPF is also known as Ormond’s disease (9).
The peritoneum is a membranous tissue that lines the abdominal cavity and covers the abdominal organs. Abdominal structures located outside of the peritoneum, known as retroperitoneal structures, include blood vessels, such as the aorta, and ureters. RPF presents with the development of aberrant chronic nonspecific fibroinflammatory tissue in the retroperitoneal space. It usually presents around the infrarenal portions of the abdominal aorta and the iliac vessels. This formation of fibrotic tissue in the majority of patients results in the entrapment and obstruction of retroperitoneal structures, such as the aorta, ureters, inferior vena cava, or other abdominal organs (2, 3).
RPF can be divided in two types: primary (or idiopathic) and secondary to other conditions. More than two thirds of cases are idiopathic, which means that the specific cause of disease is unknown. The remaining third of cases are secondary, resulting from other factors, such as infections, neoplasms, trauma, surgery, radiotherapy, and the use of certain drugs (4, 10).
Infections such as tuberculosis, histoplasmosis, actinomycosis, and syphilis can result in secondary RPF (2, 3). Various drugs, including methysergide, pergolide, bromocriptine, ergotamine, methyldopa, hydralazine, and beta blockers, as well as illegal drugs such as cocaine, can also lead to the development of RPF (9, 10). Opium abuse may be suspected as a strong risk factor for secondary RPF, as we reported in our five cases.
Recent abdominal or pelvic trauma, hemorrhage, or surgery, such as aortic bypass or anterior spinal fusion, can lead to RPF (2, 3). Idiopathic RPF can affect anyone at any age; the average age at onset of signs and symptoms is approximately 50 years. Individuals at the highest risk are men aged 40 - 60 years, with a 2 - 3: 1 male: female predominance (2). In cases associated with malignancy, the sex distribution is equal (6, 11, 12).
The systemic clinical manifestations of RPF are nonspecific constitutional symptoms, including low-grade fever, fatigue, anorexia, weight loss, and myalgia.(3, 5) Signs and symptoms of RPF may be seen as a result of entrapment and compression of retroperitoneal organs, such as the ureters, the inferior vena cava, the aorta and its branches, and the gonadal vessels (6).
Compression and obstruction of the ureters may be indicated by a dull and non-colicky pain, costovertebral angle tenderness (ureteral colic), hematuria, oliguria, anurea, and renal failure. Bilateral ureteral obstruction can present with uremic symptoms. Entrapment of the inferior vena cava can present as lower extremity edema, scrotal edema, or deep vein thrombophlebitis of the leg (1, 6, 13).
Laboratory findings in RPF are nonspecific, although anemia with a hematocrit of < 33% is common. Blood tests to check renal function, autoantibodies, and tumor markers are advised (1). Inflammatory markers (acute-phase reactants), such as ESR and CRP, are elevated in more than half of RPF patients (2, 14).
Due to bilateral ureteral compression and obstruction, renal failure can be present in 43% - 95% of cases. RPF is difficult to detect and it is important for clinicians to have sufficient awareness of this disease (1, 3). In some cases, an accurate diagnosis is made secondary to urological obstruction or renal failure (1). The diagnosis also relies strongly on radiologic findings. Radiologic imaging modalities such as X-ray, CT, magnetic resonance imaging (MRI), and positron emission tomography (PET) play an important role in making a definitive distinction between idiopathic or secondary RPF. Ultrasonography is performed as the first-line study in RPF and is useful for detecting hydronephrosis and aneurysm. Abdominal CT and MRI are the most reliable modalities for diagnosing idiopathic RPF (2, 3).
MRI can also differentiate between idiopathic RPF and malignant RPF. PET scans can be performed to evaluate disease activity, are useful for the diagnosis and for monitoring the response to treatment, and can be helpful during follow-up (3, 6). Performing a tissue biopsy remains the only way to make a definitive diagnosis, and this is required for patients with resistance to empirical corticosteroid therapy (1, 3).
The best way to treat RPF is unknown. Treatment of RPF depends on whether the underlying cause is idiopathic or secondary. There are two therapeutic strategies: medical and surgical. The treatment goal is to induce regression of the inflammatory reaction and its systemic manifestations, to stop the formation of fibrotic tissue, to improve ureteral obstruction, to relieve other retroperitoneal organs, and to prevent disease deterioration (2).
The treatment of idiopathic RPF is empirical and based on the use of corticosteroids (2, 6, 13). Induction therapy with high-dose prednisolone (1 mg/kg/day), at a maximum dose of 80 mg/kg, is recommended for 4 - 6 weeks with tapering in approximately 1 - 2 years. A reassessment of disease activity by monitoring disease features, ESR, CRP, and mass size is recommended after one month of corticosteroid therapy. After disease remission, prednisolone may be tapered to 5 - 10 mg/day in 3 - 4 months and maintained for 6 - 9 months (2, 13).
In some RPF cases in which resistance to corticosteroid treatment is seen or ureteral obstruction recurs after stopping the corticosteroid therapy, immunosuppressive agents such as azathioprine, methotrexate, mycophenolate mofetil, cyclophosphamide, and cyclosporine are advisable as adjunct medical treatments in combination with corticosteroids (2, 6, 13).
In the presence of any contraindications to corticosteroid therapy, tamoxifen (0.5 mg/kg/day) is a good alternative drug as first-line therapy. When idiopathic RPF is refractory to other treatments, biological agents can be used, including rituximab, infliximab, and tocilizumab (3, 13).
In drug-related RPF, such as in our five cases, stopping the specific causative medication is the treatment goal. In the presence of severe systemic manifestations or in cases with deterioration of disease manifestations despite discontinuation of the drug, corticosteroid therapy is recommended (2, 6). In bilateral or severe unilateral ureteral obstructions with normal renal function or refraction to medical therapy, such as in our first patient, the surgical approach is a good therapeutic choice to prevent renal damage (2, 13).
The surgical approach has no effect on disease regression and its systemic manifestations. Conservational approaches, including temporary placement of ureteral stents or nephrostomy tubes, as we used in our cases, are good therapeutic choices to correct underlying ureteral obstructions. These surgical procedures are advisable in cases with idiopathic RPF or for benign disease in patients who are poor surgical risks (2). In advanced ureteral stenosis, for which these two modalities may be ineffective, an open surgical approach, such as ureterolysis (intraperitoneal transposition and wrapping of the ureter with omental fat to provide an effective barrier against ureteral re-entrapment by fibrotic tissue, as we did in our first patient), may be necessary as an alternative procedure. Ureterolysis is an acceptable therapeutic procedure for the treatment of idiopathic RPF with or without corticosteroid therapy (2, 6).
Opium abuse may be a strong risk factor for secondary RPF. All five of our reported patients had a history of opium addiction. All of our patients used the same opium, prepared from the same region in Iran (Sistan). We suspect that impurities in certain types of opium play an important role in the pathogenesis of RPF. Some of our patients used opium again after the follow-up period; however, they used a different type of opium with a different origin, and we were surprised to see that RPF did not form again. More research needs to be carried out to prove the role of opium abuse as an etiology of secondary RPF.
3.1. Conclusion
The clinical and laboratory findings in RPF are nonspecific, and it is important to be aware of the predisposing factors for this condition, including drug abuse. We suggest that in cases with drug-induced RPF, the discontinuation of opium or changing to medical therapies may be useful and can be recommended.