1. Background
After that the first laparoscopic donor nephrectomy (LDN) was performed by Ratner et al. in 1995, it progressively became a standard method for kidney living donation (2). This technique has represented improvements in terms of cosmetic outcomes, morbidity, length of stay, and length to return to work in comparison to open surgery; all of which have promoted living donation within family and friends (3). The procedure itself has evolved over the time and technical details differ depending on regional and surgeon considerations (4).
The balance between donor health and the benefit of the recipient have to be considered in order to obtain the best outcomes for both by taking into account considerations such as the safety during the procedure as well as after the surgery (5). Vascular control is one of the most demanding steps in LDN because only one failure can cause catastrophic results; however, this procedure is relatively safe as mortality has been reported to be between 0.03% to 0.07% (4, 6, 7). Surgical techniques used to control renal vessels can be categorized into two types, the first one comprises non-transfixion methods such as simple ties or clips placed around the vessel. The second one includes sutures that can pass through the wall vessel and control the blood flow of the renal hilum. In the end, both methods can fail during the process depending on multiple variables related to technical issues (6, 8, 9).
Although it has been previously described that adverse events in vascular control can be underreported, authors e.g. His et al. in 2009 investigating the complications in the U.S. Food and Drug Administration (FDA) register found that by the time, 64% of total complications occurred when using VS, a 23% of them involved the usage of titanium clips and only 13% of total complications occurred when using non-absorbable polymer ligating (NPL) clips (6, 10). Six reports of deaths after the usage of NPL clips in 2006, forced the FDA to emit a warning that NPL clips are contraindicated for vascular control during nephrectomy (11). However, its discontinuation as part of the procedure in LDN is still controversial because as reported by Deng et al. (12) and Chan et al. (13) the usage of VS have been associated with death in two cases, and because the cost of a VS compared to NPL clips is considerably higher and it impacts directly over health systems in countries with limited resources (14).
Despite the warning released by the FDA, the controversy around using NPL clips to control renal vessels during LDN is still on debate as authors such as Simforoosh et al. (15) and Janki et al. (4) show that even though there are some concerns to the use of these devices, it is an option in certain settings and its utilization can be considered safe when it is performed by experienced surgeons (16).
2. Objectives
We considered that this paper will contribute to this discussion as it shows the safety during our 500 LDN cases experience when using NPL clips as the main method for vascular control in one transplantation center.
3. Methods
A total of 500 LDNs were performed in our center from July 2003 to July 2017. The data were obtained retrospectively from the records of clinical registers. For each patient, operative time, blood loss, length of hospital stays, and early complications were estimated. In all procedures, vascular control was done using two NPL clips in each vessel or a VS. Also, NPL clips were used for both artery and renal vein in left-sided nephrectomies; when nephrectomy was performed on the right side, two NPL clips were only used in the renal artery and a VS was used for the renal vein as it allows to be extended and a small portion of vena cava to be incorporated into the vascular staple line. Complications were defined as adverse events within the peri-operative period that altered patient recovery, prolonged hospital stay or represented technical deviations during the surgical procedure.
3.1. Analysis
Frequencies and percentages were used for categorical variables. The distribution of the numerical variables was assessed using the Shapiro-Wilk test, and the variables were reported as medians and interquartile ranges (IQR) used for descriptive statistics. The analysis of the perioperative variables was performed using Pearson’s chi-square test for categorical variables and the Mann-Whitney non-parametric test for numerical variables. Comparisons between NPL clip and VS groups were performed for donor characteristics and perioperative variables.
4. Results
4.1. Donor Characteristics
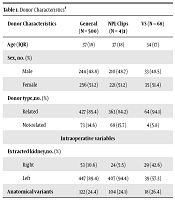
A total of 500 donor hand-assisted laparoscopic nephrectomies were performed from July 2003 to July 2017. Extracted kidneys were left in 89.2% (n = 446) of cases; 51.2% of donors were female (n = 256); the age of donor population ranged from 18 to 73 years with a median of 37 years (IQR = 18). Eighty-five percent of donors were related to recipients (n = 427) and 24.4% of the patients (n = 122) had more than one vein or artery (see Table 1).
4.2. Perioperative Variables
The surgical incision was Phanestiell in 87.4% of donors (n = 436) and infraumbilical midline for the remaining was 12.6% (n = 63). The median surgical time was 2 hours (IQR = 0.5) (range of 1 - 4.5 hours). The warm ischemia time was 3 minutes (IQR = 1) (range of 1 - 20 minutes). Median intraoperative bleeding was 50 cc (IQR = 50) with maximum bleeding of 3000 cc in a case where the Aorta artery was injured at the ostium renal artery. The median length of hospital stay was 2 days (n = 397), while the longest hospitalization was 15 days.
| Donor Characteristics | General (N = 500) | NPL Clips (N = 431) | VS (N = 68) |
|---|---|---|---|
| Age (IQR) | 37 (18) | 37 (18) | 34 (17) |
| Sex (%) | |||
| Male | 244 (48.8) | 210 (48.7) | 33 (48.5) |
| Female | 256 (51.2) | 221 (51.2) | 35 (51.4) |
| Donor type (%) | |||
| Related | 427 (85.4) | 363 (84.2) | 64 (94.1) |
| Not-related | 73 (14.6) | 68 (15.7) | 4 (5.8) |
| Intraoperative variables | |||
| Extracted kidney (%) | |||
| Right | 53 (10.6) | 24 (5.5) | 29 (42.6) |
| Left | 447 (89.4) | 407 (94.4) | 39 (57.3) |
| Anatomical variants | 122 (24.4) | 104 (24.1) | 18 (26.4) |
Donor Characteristicsa
Vascular control was performed with VS in 68 patients (13.6%) and with NPL clips in 431 patients (86.3%); only 1 patient (0.2%) required manual suture which was not included in the comparative analysis. Sociodemographic characteristics of the patients in both groups were similar. The median surgical time and length of hospital stay did not vary between the groups (2 hours; 2 days) (P = 0.34; 0.53), median of warm ischemia time was 3 minutes (IQR = 1) in the NPL clip group and 4 minutes (IQR = 1) in the VS group and median bleeding was 50 cc in both groups (P = 0.84) (Table 2).
Eight patients (1.6%) required conversion to open surgery of which 5 were done electively due to technical difficulties because patients had intraperitoneal adherence, 2 conversions were made due to bleeding secondary to splenic capsule injury, and one additional secondary to the renal artery rupture described. Out of the 8 patients who had a surgical conversion, 5 were presented in the NPL clip group and 3 in the VS group (1.1% and 4.4%, respectively). The rate of conversion to open surgery was significantly lower in NPL clip group (P = 0.04). The overall complication rate was 6.8% (n = 34) among 15 donors had a vascular injury and none of those were related to vascular control devices. The proportion of complications in each group was 6% (26/431) in the NPL clip group and 11.7% (8/68) in the VS group. There were no donor complications related to the use of the NPL clip, no transfusions, no renal vessel injuries, and no cases of clip dislodgement, slippage or bleeding (Table 2).
| Outcomes | General (N = 500) | NPL (N = 431) | VS (N = 68) | P Value |
|---|---|---|---|---|
| Operating room time, hours (IQR) | 2 (0.5) | 2 (0.5) | 2 (0.65) | 0.34 |
| Warm ischemia time, minutes (IQR) | 3 (1) | 3 (1) | 4 (1) | 0.00 |
| Bleeding, cc (IQR) | 50 (50) | 50 (50) | 50 (50) | 0.84 |
| Conversion (%) | 8 (1.6) | 5 (1.1) | 3 (4.4) | 0.04 |
| Length of hospital stay, days (IQR) | 2 (0) | 2 (0) | 2 (0) | 0.53 |
| Complications (%) | 34 (6.8) | 26 (6) | 8 (11.7) | 0.34 |
Comparison of Perioperative Variables
5. Discussion
As LDN has become a common procedure in kidney transplantation because of its benefits, representing better cosmetic results, shorter hospital stay, and less convalescence time; thus the consequences or possible complications have been studied widely (3). Total complication rates described by various papers range from 3 to 9% (7, 17, 18) of which the mortality can be considered the biggest concern because donors are totally healthy patients prior to the surgery (19). This proposition explains the controversy created by the issues raised over vascular control during LDN in 2006 by Friedman et al. (20), in which authors argued that the usage of NPL clips is an important factor for fatal complications after LDN. Even though the FDA issued a class II recall over utilizing NPL clips during LDN, multiple papers are presenting positive experience with no mortality cases, cost-effectiveness and technical advantages (longer renal artery and vein segments) supporting the idea that the discussion is not closed (16, 21-23). For these reasons, this study aimed to describe a single-center experience over 500 cases using NPL clips as the main method for vascular control during LDN.
Controversy about the best method to control vascular hilum during LDN started in 2006 when Friedman et al. (20) reported results from a survey in which 213 surgeons from EEUU expressed their experience with the use of staplers, non-locking clips, and locking clips. This paper showed surgeons’ concerns about the use of non-traxfixing options, causing an investigation by the FDA and the manufacturer of Hem-o-lok clips (Teleflex), concluding that by the time, there were three cases of deaths related to its use. The investigation motivated the manufacturer (Teleflex) to issue a Class II recall that contraindicated its use in LDN (24). However, there are some arguments to clarify the controversy. Firstly, the publication was based on a survey, which means a possibility of bias. Secondly, deaths reported by the publication were related to non-locking clips rather than locking clips and finally, when evaluating surgeons’ opinion about the safety, they rated equally the usage of VS and NPL clips for vascular control (20). Furthermore, after FDA investigation, there was no difference whether the error, which caused associated deaths, was device- or user-related (25).
The second paper published by Friedman et al. (1) reported that between three to six additional fatalities related to Hem-o-lok had occurred since 2006 recall. Although the use of VS is the standard technique in most centers of the United States, the safety of NPL clips for laparoscopic nephrectomy has been assessed in several publications. Its safety is supported by 12 publications and 5369 cases along with this report, including any kind of nephrectomy and no deaths or major complications occurred (14, 15, 22, 23, 25-32). Out of those 5369 nephrectomies, 3840 were LDN and the summary of these papers is shown in Table 3.
| Authors | Year | Number of Cases | Deaths | Purpose |
|---|---|---|---|---|
| Eswar and Badillo (28) | 2004 | 50 | 0 | Ablative |
| Kapoor et al. (29) | 2006 | 246 | 0 | Ablative |
| Modi et al. (22) | 2009 | 24 | 0 | Ablative |
| Baldwin et al. (30) | 2005 | 50 | 0 | LDN |
| Kaushik et al. (31) | 2006 | 106 | 0 | LDN |
| Baumert et al. (32) | 2006 | 130 | 0 | LDN |
| Ay et al. (23) | 2010 | 367 | 0 | LDN |
| Ye et al. (26) | 2010 | 109 | 0 | LDN |
| Goh et al. (27) | 2014 | 23 | 0 | LDN |
| van der Merwe and Heyns (14) | 2014 | 43 | 0 | LDN |
| Simforoosh et al. (15) | 2014 | 1510 | 0 | LDN |
| Ponsky et al. (25) | 2008 | 1695 | 0 | DV-486 / ablative - 1209 |
| Current report | 2018 | 431 | 0 | LDN |
| Total cases | 4784 | 0 |
Summary of Papers Published Since 2004 Addressing NPL Safety During Nephrectomy
There are additional advantages to be considered in favor of NPL clips (31). The length of the renal vein and artery obtained after the procedure are up to 5 mm longer when using NPL clips, having a possible impact on the difficulty of the transplantation and consequently, over post-transplant outcomes (16, 30, 31, 33, 34). Additionally, in laboratory research published by Elliot et al. (35) in 2005, it was demonstrated that bursting pressures for NPL clips were over physiologic artery pressures (1220 - 1500 mmHg) in comparison to the bursting pressure found for VS (262 mmHg). Cost-effectiveness is another relevant consideration for this controversy, especially in countries with limited economic resources as the difference in cost range from 253 to 1077 USD per patient and it has clearly described NPL clip superiority on this topic (Table 4). Janki et al. (4) believe that costs should be considered the secondary issue; however, as Simforoosh et al. (15) described, it should also be tempered the fact that in a series of 1510 LDN cases, savings could reach an amount of 1.36 million USD. In our research, the cost per patient of NPL clip was 79 USD and VS was 350 USD.
Costs Comparison Between NPL Clips and VS for Vascular Control in LDN
All benefits mentioned previously are paramount to maintain donor safety and ensure continued success of living kidney donor programs (38). For this reason, it is important to establish some points during the utilization of NPL clips that ensure the safety of the procedure, which include the use of two clips; sparing 2 to 3 mm of the renal artery/vein distal to the clips and applying NPL clip a few millimeters away from the aortic root of the renal artery to avoid a probable risk of pseudoaneurysm (16, 21). Observation of the locking tip of the clip around the vessel before final deployment, the tactile feedback, and the peculiar clicking sound of the locked jaw at the time of application are also important and can make this device user-friendly and safe (22, 25, 30). Additionally, the maintenance of the instruments used for NPL clip deployment must be performed periodically in order to guarantee the adequate action of the jaws (39).
Although the real rate of complications for both VS and NPL clips could be under-registered, the use of VS has been associated with malfunction in up to 1.7% of the cases and rates of dysfunction as high as 66% within laparoscopic surgeons (10-12, 30, 31). Complications reported to the Manufacturer and User Facility Device Experience (MAUDE) during LDN were studied by Hsi et al. (8) in 2007, showing that out of 2172 events correlated with the total nephrectomy- or kidney-related reports, 352 events were associated with the device used for the renal hilum control during laparoscopy and from those 63% (223 complications) were identified with VS and 5% (18 complications) with NPL clip. Results from a systematic review published by Liu et al. (40) in 2018 in which 32145 patients were included, it was found that there were no significant differences regarding the rate of failure, death rate, and severe hemorrhage rate. However, when comparing the cost of using VS or NPL clip, there were significant differences in favor of NPL clip because its costs are 10 times lower than VS (40). These pieces of evidence keep pointing to evaluate some considerations about the decision of using VS or NPL clip as methods for controlling the vascular hilum during LDN.
To the best of our knowledge, there are no clinical trials published addressing this issue and most papers are retrospective; however, we believe our findings could be improved in future research. Firstly, the information bias could have affected the results since it was a retrospective report. Secondly, our study focused on early complications after the procedure, but long-term outcomes were not described. This decision was made on the basis that the great majority of complications in a living donor occurs during the first week.
5.1. Conclusions
The best ideal method for vascular control at donor nephrectomy is still controversial (23). However, as there were no major bleeding episodes or donor losses caused by NPL clips at intraoperative and early postoperative periods in any of the cases who had undergone LDN with the two methods, our results support the advantages, safety, and low cost of the use of NPL to control renal vessels during laparoscopic nephrectomy.
As highlighted by other authors, including Liu et al. (40), it is paramount to balance opinions when considering this controversy before the national policies are established, especially in countries with limited resources as it is not clear the real differences of using NPL clips or VS in terms of clinical outcomes, but NPL clips have a favorable difference in terms of costs (14, 25). We also consider that surgeons’ experience is essential for adequate use of NPL clips as there are some requirements in terms of surgical technique in order to achieve successful results.