1. Background
Several studies have investigated chronic constipation prevalence in the general population, reported it as much as 5% to 15%. Different definitions for constipation, cultural, ethnic, and lifestyle variations are accounted for this diversity (1). The condition is more prevalent in the female gender, elderly, and lower socioeconomic status (2). Nevertheless, increasing life expectancy may result in more prevalence of constipation in the future (3).
A systematic review reported the prevalence rate of constipation in different regions. It was 26.8 - 28% in South America, 4.4 - 30.7% (median 19.7%) in Oceania, 0.7 - 79% (median 19.2%) in Europe, 3.2 - 45% (median 16%) in North America, 1.4 - 32.9% (median 10.8%) in Asia, and 29.2% in South Africa, according to a solitary study of this region (4).
Constipation can affect patients' quality of life and even influence their behavior. The patients feel lower healthiness and experience depression more than people without constipation (5).
Women with constipation worry about choosing food and clothes and have difficulties in their interactions (6).
The economic burden of constipation is notable. In the United States, 235,000,000 $ was spent annually on the diagnosis and treatment of constipation (7). The number of inpatient discharges for constipation was 21,190 in 1997 compared to 48,450 in 2010. Also, related costs have been increased significantly between these two years (8). Because of chronicity and symptom indefiniteness, the constipation burden for the health system is substantial (9).
Thirty-five percent of patients with functional bowel disorders use complementary and alternative medicine, whereas 32.1% of patients with constipation use CAM methods (10). Different modalities of complementary and alternative therapies, including herbal medicine, massage, acupuncture, and moxibustion have been examined in many studies. It seems that acupuncture and herbal medicines are the most effective CAM modalities for constipation (11). Many herbal medicines are used traditionally for gastrointestinal disorders (12). "Jalinous" capsule" is a Persian medicine preparation, which is a combination of rose (Rosa damascena), mastic (Pistacia lentiscus), Aloe vera (Aloe barbadensis), and turpeth (Ipomoea turpethum). This formula has been recommended for the treatment of constipation in “Qharabadin Azam”, one of the important Qarabadins (pharmacopeia) in Persian medicine (13). Prolonged use of laxatives weakens the bowel and decreases its function, but this formula contains gradients (e.g., rose and mastic) that correct these side effects and strengthen the stomach and bowel; meanwhile, excrete waste material not only from the bowel but also from all body.
2. Objectives
The aim of this study was to evaluate the efficacy of "Jalinous" capsule in adult functional constipation.
3. Methods
3.1. Study Design
This study was a randomized, double-blind, and placebo-controlled study. The Ethics Committee of Shiraz University of Medical Sciences reviewed and approved the protocol of the study (registration number: IR.SUMS.MED.REC.1397.471). IRCT code of this study is IRCT20190312043025N1. Eligible subjects were recruited through a questionnaire based on the Rome IV criteria and obstructed defecation syndrome (ODS) questioner. The diagnosis of constipation was confirmed by a gastroenterologist. Follow-up visits were done at the 2nd and 4th weeks of treatment and 4 weeks after treatment withdrawal. The sample size was 126.
3.2. Participants
The patients were recruited by purposeful convenience sampling from Imam Khomeini hospital's gastrointestinal disease clinic of Tehran University of Medical Sciences. The inclusion criteria were: 1- Age 18 to 50; 2- Ability to understand, speak, and answer the questions; 3- Qualification for ROME IV criteria for constipation; 4- Confirmation of diagnosis by a gastroenterologist; 5- Not pregnant or lactating (for women).
The exclusion criteria were: 1- Use of laxatives other than study medication more than twice a week during the study; 2- Recent use of medicine can induce constipation; 3- Previous history of any abdominal and gastrointestinal surgery; 4- New-onset disease that may lead to hospitalization; 5- History of allergy to Aloe vera, rose, Indian jalap, or mastic; 6- Organic disease alarm signs (rectal bleeding, fever, loss of appetite, weight loss, etc.).
3.3. Randomization and Blinding
Patients eligible for inclusion criteria were randomly assigned into two groups, including Group A: Jalinous capsule and psyllium sachet and group B: placebo capsule and psyllium sachet using balanced block randomization with Excel computer software.
Drugs were packaged into two groups (A and B) by a person who did not participate in the study. Patients, physicians, and all persons who participated in collecting data were blind to the allocation of the patients. After data analysis, the contents of the package were disclosed.
3.4. Interventions
Patients in both groups were administered sachets of psyllium granules (10 g, in a glass of water) every morning. Psyllium sachets were manufactured by Dineh Co., made from plantago psyllium pellet seeds. Patients in the intervention group were administered Jalinous capsules (two capsules at bedtime) with warm water, whilst patients in the control group took placebo capsules (two capsules at bedtime) with warm water. "Jalinous" capsules contained mastic, Aloe vera, rose, and Indian jalap powder were produced by Behpad Co, under the license of natural, traditional, and supplement department of Iranian Food and Drug Administration (registration number: 93- 0212). Placebo capsules contained starch powder. Both groups were advised to take resources of fiber sufficiently and enough liquids.
Patients were treated for four weeks and were followed up for four weeks later; indeed, a physician visited them every other week.
3.5. Patient’s Assessment
Symptoms of constipation were assessed using an investigator-generated questionnaire based on the Rome IV criteria and ODS questioner. Bristol stool scale was used to specify the stool form.
Primary outcome measurements are frequency of defecation, percentage of incomplete defecation and evacuation, straining during defecation, using manual maneuver to facilitate evacuation and defecation time. Secondary outcome measurements are stool consistency, rectal pain, or rectorrhagia during defecation, quality of life impairment, overall self-reported improvement in symptoms after the treatment, and reported side effects.
3.6. Safety Assessment
Possible adverse effects of the prescribed drugs were evaluated by CTCAE criteria (Common Terminology Criteria for Adverse Events). The frequency and severity of the symptoms and the relationship with interventions were assessed. The severity was defined as mild (easily tolerated), moderate (enough discomfort to interfere with usual activity), severe (inability to perform usual activities).
3.7. Statistical Analysis
The sample size was estimated at 53 per group based on earlier experience and the pre- and post-intervention standard deviations of 0.65 (SD) in order to reach a mean difference (reduction in defecation per week) of 0.36 (D) with the following specifications and using the sample size equation (N = [(Z1-α/2 + Z1-β)2 × SD2]/D2) for comparing two means; the estimated sample size was increased to 65 per group to take account of potential attrition 20% (α = 0.01; β = 0.1). We used the Kolmogorov-Smirnov test to test whether data were normally distributed. Descriptive baseline characteristics for two groups’ comparisons were tabulated as mean ± SD, median (inter-quartile range), or as percentages. Comparing between two groups for categorical data were statistically analyzed using chi-square or Fisher's exact test and for continuous data were statistically analyzed using t-test and Mann-Whitney U test. The primary efficacy data on nausea and vomiting were examined using intention-to-treat analysis. Using General Linear Model (GLM) score, outcomes were compared between the two groups by repeated measures ANOVA test. The primary efficacy was examined using intention-to-treat analysis. Additionally, we used a generalized estimating equation (GEE) model to estimate the differences in values of endpoints at each time point between the two groups (between-group effects). Within-group effects were assessed with the Friedman test and Wilcoxon test. A p-value of 0.05 or less was considered statistically significant. Data were analyzed using IBM SPSS statistics version 22 and stata version 14.
4. Results
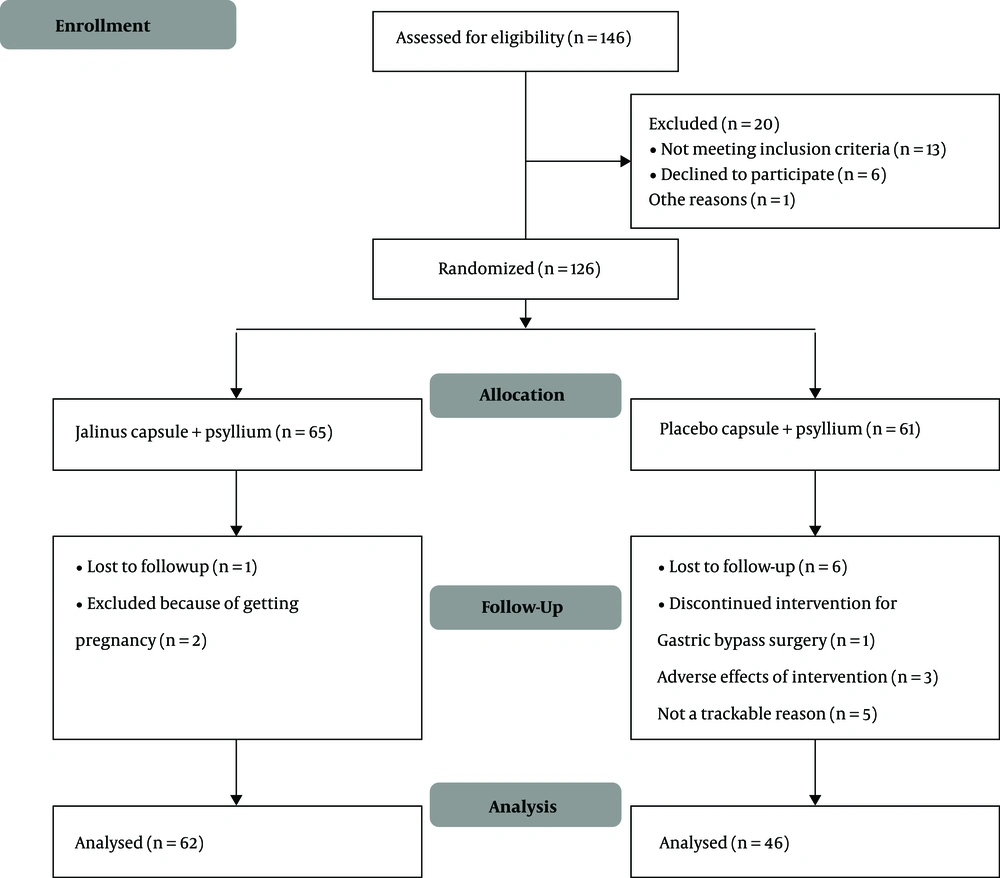
One hundred and forty-six patients with a diagnosis of constipation who admitted to the gastroenterology clinic were evaluated from May to December 2019, according to the inclusion criteria. One hundred and twenty-six patients, who were eligible for the study and signed the informed consent, were recruited. They were randomized blindly and allocated to either intervention (n = 65) or control groups (n = 61). Finally, 62 patients were analyzed in the intervention group and 46 patients in the control group. The process of the study is presented in the flow diagram (Figure 1).
Demographic properties and general characteristics of patients are summarized in Table 1. There was no statistically significant difference between groups in baseline demographic properties (Table 1).
4.1. Constipation Symptoms Changes
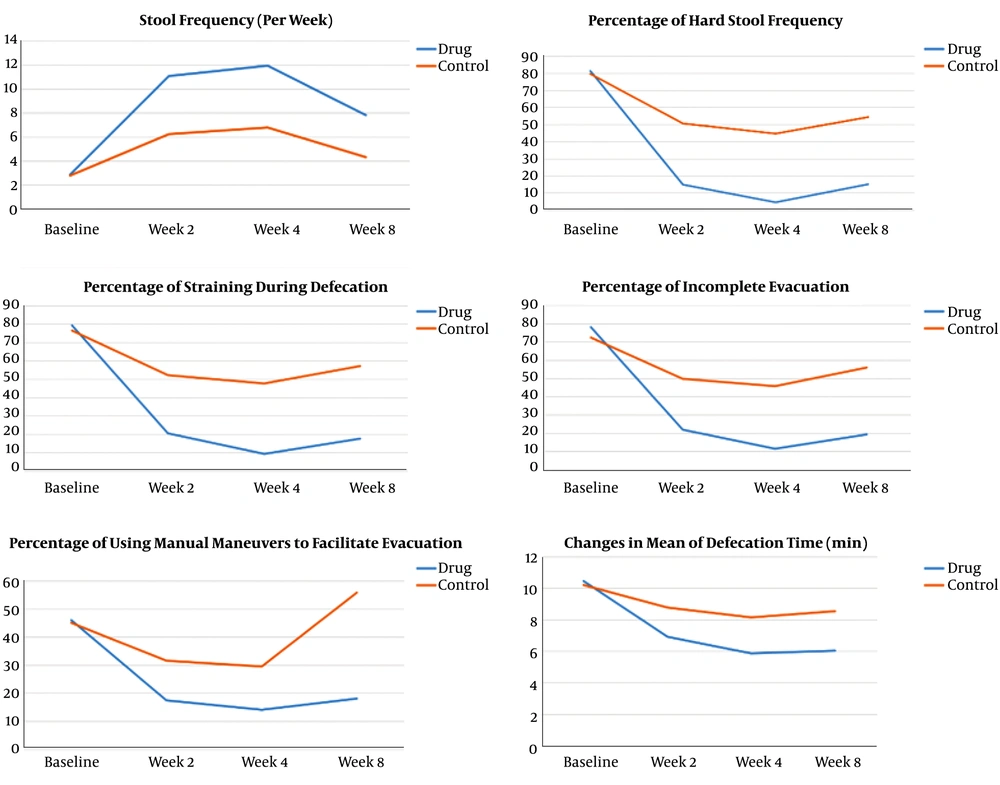
Baseline variables, including defecation frequency, percentage of hard stools, percentage of straining during defecation, percentage of incomplete evacuation, percentage of manual maneuver to facilitate evacuation, and defecation time demonstrated no significant difference between the groups. In subsequent measurements, all of these variables showed significant improvement in both groups (P < 0.001), but these improvements in the intervention group were significantly more than in the control group (P < 0.001) (Table 2). Symptom changes over time are illustrated in Figure 2.
| Group | Time | Effect | |||||
|---|---|---|---|---|---|---|---|
| Baseline | Week2 | Week 4 | Week 8 | Within-Group b | Between-Group c | Adjusted Between-Group c | |
| Defecation frequency per week d | P < 0.001 | P < 0.001 | |||||
| Drug | 2.91 (1.77) | 11 (4.6) | 11.95 (5.35) | 7.85 (2.82) | P < 0.001 | ||
| Control | 2.83 (1.69) | 6.28 (3.88) | 6.83 (4.18) | 4.35 (1.92) | P < 0.001 | ||
| Percent of hard stool d | P < 0.001 | P < 0.001 | |||||
| Drug | 81.45 (15.98) | 15 (18.15) | 4.67 (8.81) | 15.19 (12.83) | P < 0.001 | ||
| Control | 79.78 (13.90) | 50.76 (23.68) | 44.89 (25.57) | 54.45 (23.67) | P < 0.001 | ||
| Percent of Straining during defecation d | P < 0.001 | P < 0.001 | |||||
| Drug | 79.35 (16.58) | 20.64 (18.52) | 9.51 (15.16) | 17.82 (17.12) | P < 0.001 | ||
| Control | 76.52 (17.53) | 52.17 (23.56) | 47.71 (27.23) | 57.17 (26.2) | P < 0.001 | ||
| Percent of incomplete evacuation d | P < 0.001 | P < 0.001 | |||||
| Drug | 78.22 (17.60) | 21.93 (21.41) | 11.61 (15.41) | 19.35 (17.95) | P < 0.001 | ||
| Control | 72.60 (17.78) | 49.78 (24.60) | 45.76 (26.37) | 55.97 (25.07) | P < 0.001 | ||
| Percent of Manual maneuver to facilitate evacuation d | P < 0.001 | P < 0.14 | |||||
| Drug | 46.12 (37.91) | 17.58 (29.44) | 14.27 (27.67) | 18.22 (28.77) | P < 0.001 | ||
| Control | 45.21 (43.19) | 31.73 (39.34) | 29.67 (39.81) | 35.97 (39.88) | P < 0.001 | ||
| Defecation time (min) | P < 0.001 | P < 0.001 | |||||
| Drug | 10.48 (5.84) | 6.94 (3.55) | 5.89 (2.13) | 6.05 (2.24) | P < 0.001 | ||
| Control | 10.22 (4.94) | 8.80 (4.74) | 8.15 (4.13) | 8.56 (4.08) | P < 0.001 | ||
Tests of Within- and Between-Subject Effects of Treatment Over the Study Period in the Study Groups a
4.2. Stool Consistency
In weak two, four, and eight, the patients in the intervention group had less frequent hard stool form than in the control group (P < 0.001) (Table 3).
| Time and Stool form b | Drug | Control | P Value c |
|---|---|---|---|
| Baseline | _ | ||
| Hard stool | 62 (100) | 46 (100) | |
| Normal | 0 (0.0) | 0 (0.0) | |
| Loose stool | 0 (0.0) | 0 (0.0) | |
| Week 2 | < 0.001 | ||
| Hard stool | 3 (4.8) | 25 (54.3) | |
| Normal | 38 (61.3) | 19 (41.3) | |
| Loose stool | 21 (33.9) | 2 (4.3) | |
| Week 4 | < 0.001 | ||
| Hard stool | 0 (0.0) | 18 (39.1) | |
| Normal | 31 (50.0) | 25 (54.3) | |
| Loose stool | 31 (50.0) | 3 (6.5) | |
| Week 8 | < 0 .001 | ||
| Hard stool | 1 (1.6) | 30 (65.2) | |
| Normal | 52 (83.9) | 16 (34.8) | |
| Loose stool | 9 (14.5) | 0 (0.0) |
Comparison of the Changes in Stool form Between Groups a
based on the Bristol stool scale.
4.3. Rectal Pain or Rectorrhagia During Defecation
Rectal pain or rectorrhagia showed a significant improvement in both groups (P < 0.001 and P = 0.02 in drug and control groups, respectively). This improvement was more prominent in the intervention group; however, it was not significant statistically (Table 4).
4.4. Quality of Life Impairment
As shown in Table 5, the quality of life showed a significant improvement in both groups (P < 0.001), while the improvements in the intervention group were significantly more than in the control group (P = 0.007) (Table 5).
The Quality of Life Impairment Severity in Both Groups Before and After the treatment
4.5. Patients’ Self-reported Improvement in Symptoms
Patients in the intervention group felt more improvement in symptoms than patients in the placebo group (P < 0.001) (Table 6).
| Pt Improvement Sense | Drug Group | Control Group | Total |
|---|---|---|---|
| No improvement | 1 (1.6) | 22 (48.9) | 23 (21.5) |
| Partial improvement | 26 (41.9) | 18 (40.0) | 44 (41.1) |
| Complete improvement | 35 (56.5) | 5 (11.1) | 40 (37.4) |
4.6. Adverse Effects
Reported adverse effects of interventions were: 1- Intestinal colicky pain (three cases in the intervention group and one case in the placebo group), which lasted 1 to 10 days. 2- Abdominal distention (three cases, all out of the intervention group) lasted 1 to 3 days. 3- Fullness sensation in stomach (two cases out of the intervention group). The severity of all of the above side effects was mild, and treatment continued.
During the interventional period, apart from the laxative effect, some patients experienced some positive effects, including a significant reduction in the severity of chronic wheal, improvement of heartburn and gastroesophageal reflux, dysmenorrhea improvement, and flank pain elimination.
5. Discussion
In the current study, traditional Persian medicine was examined for functional constipation.
"Jalinous" capsule along with psyllium was shown to be more effective than placebo plus psyllium in the treatment of constipation and could subside its symptoms more successfully.
Although constipation is a common and prevalent disorder, its management continues to pose a significant health problem. Integration of complementary and alternative medicine into the current health system may result in beneficial outcomes, but the absence of accurate randomized placebo-controlled trials on many of these modalities is a serious limitation. Therefore, such studies about the efficacy and safety of CAM remedies will be of value (14, 15).
Herbal medicines are among the most commonly used alternative therapies for constipation in adults and children, and most studies have demonstrated their effectiveness in treating the condition; however, low-quality studies are frequent (11, 16).
In addition to herbal remedies, other alternative therapies have been studied to treat constipation. Acupuncture or electroacupuncture was found to be effective in treating constipation (11), versus moxibustion (11), massage (11, 17), and reflexology (18), which their findings are inconclusive. Anyway, more accurate studies are needed.
In Persian medicine, lifestyle modification is the first step toward constipation treatment (19). Then if it was not sufficient, herbal therapies are considered (20). "Jalinous" is a preparation suggested in Persian medicine textbooks (21).
Aloe vera is the main component of many powerful purgative drugs in Persian medicine (22).
It is a stimulant laxative, which may act through anthraquinones. They improve defecation frequency and stool consistency (11). Aloe-emodin is accounted for purgative properties of Aloe vera. It is the product of Aloin metabolism by colonic flora (23). Odes and colleagues performed a randomized controlled trial on 35 patients with chronic constipation with a compound drug-containing Chelidonium majus, Aloe vera extract, and psyllium compared with placebo. In the drug group, bowel motility increased and stool consistency decreased (24) more than the placebo group. Recent studies have demonstrated many effects for Rosa damascene. It has antibacterial, antioxidant, anticancer, anti-inflammatory, analgesic, astringent, neuroprotective, cardioprotective, gastrointestinal, and hepatic effects (25, 26).
It is a considerable remedy in traditional medicine and a cost-effective herbal therapy with curative utilization in modern medicine (27). In Persian medicine, Rosa damascene is considered a gentle safe laxative and even can be prescribed for children and pregnant women (19, 28). If administered with proper therapeutic dosage, no side effects are expected (29).
Nowadays, mechanisms of action are described for Rosa damascene: osmotic infiltration of fluids into the intestine (30) and stimulatory laxative effect (28).
Mastic (Pistacia lenticus) is used traditionally to treat sore throats, coughs, eczema, gastric pain, renal stones, and jaundice (31). In addition to anti-inflammatory (32), hepatoprotective (33), wound healing (34), etc., Pistacia lenticus has digestive effects. A three weeks administration of Pistacia lenticus resin resulted in ameliorating some digestive symptoms such as stomachache and heartburn, rather than placebo (35) turpeth (Ipomoea turpethum) is used for its purgative, hepatoprotective, antihelminthic, anti-inflammatory, expectorant, and antipyretic effects traditionally (36) In mice, the extract of Ipomoea turpethum led to a significant improvement of percentage of wet feces and intestinal motility in the treated groups compared to the negative control group. Although it made no significant alterations in the intestinal content volume, turpeth showed potent laxative effects (37).
Several herbal medicines, single or combining, have shown significant effects on constipation. As different etiologies have been described for functional constipation, prescribing a combination of different herbal medicines may result in the employment of different mechanisms of action. In addition, combination therapy with less amount of ingredients decreases their side effects by prolonged use. Moreover, it may contain herbal remedies other than laxatives, which can relieve adverse effects of stimulant laxatives (11). Nevertheless, polyherbal drugs may cause more side effects. In the current study, despite careful monitoring, no serious adverse effects were reported. Furthermore, as was reported in the results, it had other gastrointestinal (GI) and non-GI effects that should be taken into consideration in future studies.
5.1. Conclusion
This study showed that "Jalinous" capsule is an effective and safe treatment for functional constipation in adults, but more studies are needed to confirm this finding. Since no clinical study had been carried out about "Jalinous" capsule, ethically we could deprive no patient of standard treatment. So all participants received psyllium. Future studies can consider the evaluation of exclusive prescription of "Jalinous" and its effects on other GI and non-GI symptoms.