1. Background
Irrational medication use, as a global challenge to healthcare systems worldwide, especially hospitals, is reviewed by Drug Utilization Evaluation programs. Appropriate medication use is an issue of considerable concern owing to limited medical and financial resources, particularly in developing countries. The World Health Organization has suggested clinical practice guidelines as a regulatory strategy to improve the drug utilization pattern (1). Implementation of clinical practice guidelines will enhance medication consumption through minimizing inappropriate medication prescription, decreasing costs, and managing medication supplies (2, 3). Human albumin, one of the circulating blood proteins determining oncotic pressure and regulating body fluid distribution, which also carry substances such as hormones, bilirubin, and drugs, is limited in resources and high in costs (4). Human albumin use has been widely accepted indications such as therapeutic paracentesis, plasmapheresis, spontaneous bacterial peritonitis, and hepato-renal syndrome, as well as several more indications justified only if specific criteria are met (5). Regarding the broad spectrum of accepted human albumin indications, irrational utilization of albumin in some clinical settings is prevalent. The previous research found that nutrition support and hypoalbuminemia were the most common inappropriate uses of albumin in our country (6). Previous studies in Iran showed that the percentage of inappropriate use of albumin is relatively high (7). Irrational albumin use in Iran is reported as high as 70% or more in previous studies (8, 9), and it is also reported that inappropriate albumin prescription could amount for 88.6% additional costs (10). It could be expected adherence to standard guidelines minimize the inappropriate use of albumin.
2. Objectives
Considering the high cost and limited resources of human albumin, our study aimed to assess the impact of the guideline implementation and clinical pharmacist’s intervention on the medication prescription and consumption pattern in the Loghman-Hakim Teaching Hospital in Iran.
3. Methods
3.1. Study Design
This interventional study was conducted in the Loghman-Hakim Hospital from May 2017 to January 2018. Loghman-Hakim Hospital is a general tertiary educational hospital with 420 beds, 24 wards, 33 clinic units, and 14 paraclinical units affiliated with Shahid Beheshti University of Medical Sciences, Tehran, Iran, which was constructed in 1971. The study was approved by the Hospital Medical Ethics Committee and Institutional Review Board (IR.SBMU.PHARMACY.REC.1397.013), and written informed consent was obtained from the patients or their family members. One hundred patients who were candidate to receive albumin were included in the period of study. Inclusion criteria were as follows: patients hospitalized in internal, emergency, intensive care unit (ICU), neurosurgery and general surgery wards, who were candidates to receive albumin. In the pre-intervention phase, in a 5-month period, information about albumin prescription was gathered and then in the post-intervention phase, in a 8-month period, prescription was limited only after consultation about the indication based on the guidelines by clinical pharmacist’ supervision.
Based on medication ABC analysis in Loghman-hakim hospital, Albumin categorized as A category in the hospital in 2017 and 2018 and human albumin solution is determined as one of the top ten most-costly medication in the hospital.
3.2. Indications Guidelines
A guideline to use albumin was designed by a clinical pharmacist by exploiting relevant international guidelines from 2010, references and textbooks as well as clinical trials and case reports on effects of albumin consumption on clinical course of patients to cover all albumin indications. Then indications were classified as “absolute”, “may be beneficial” and “should not be used”. Afterward, it was reviewed by the hospital medical team in a joint meeting for final revision. The final version of Loghman-Hakim albumin indications guideline was released to be used by the medical team, and albumin was prescribed only by performing consultation of hospital clinical pharmacists based on the guidelines, thereafter. The medical team is included in, deputy of education, head of hospital wards and intensive care, clinical pharmacy, nephrology, gastroenterology, internal medicine, neurology, neurosurgery and surgery specialties. Supplementary file appendix 1 provides the Loghman-Hakim albumin indication guidelines.
3.3. Guidelines Implementation
In the pre-intervention phase of the study, all wards were allowed to prescribe and receive albumin according to the physician’s order with no limitation and consultation, to explore prescriptions pattern and data gathered on inappropriate medication use.
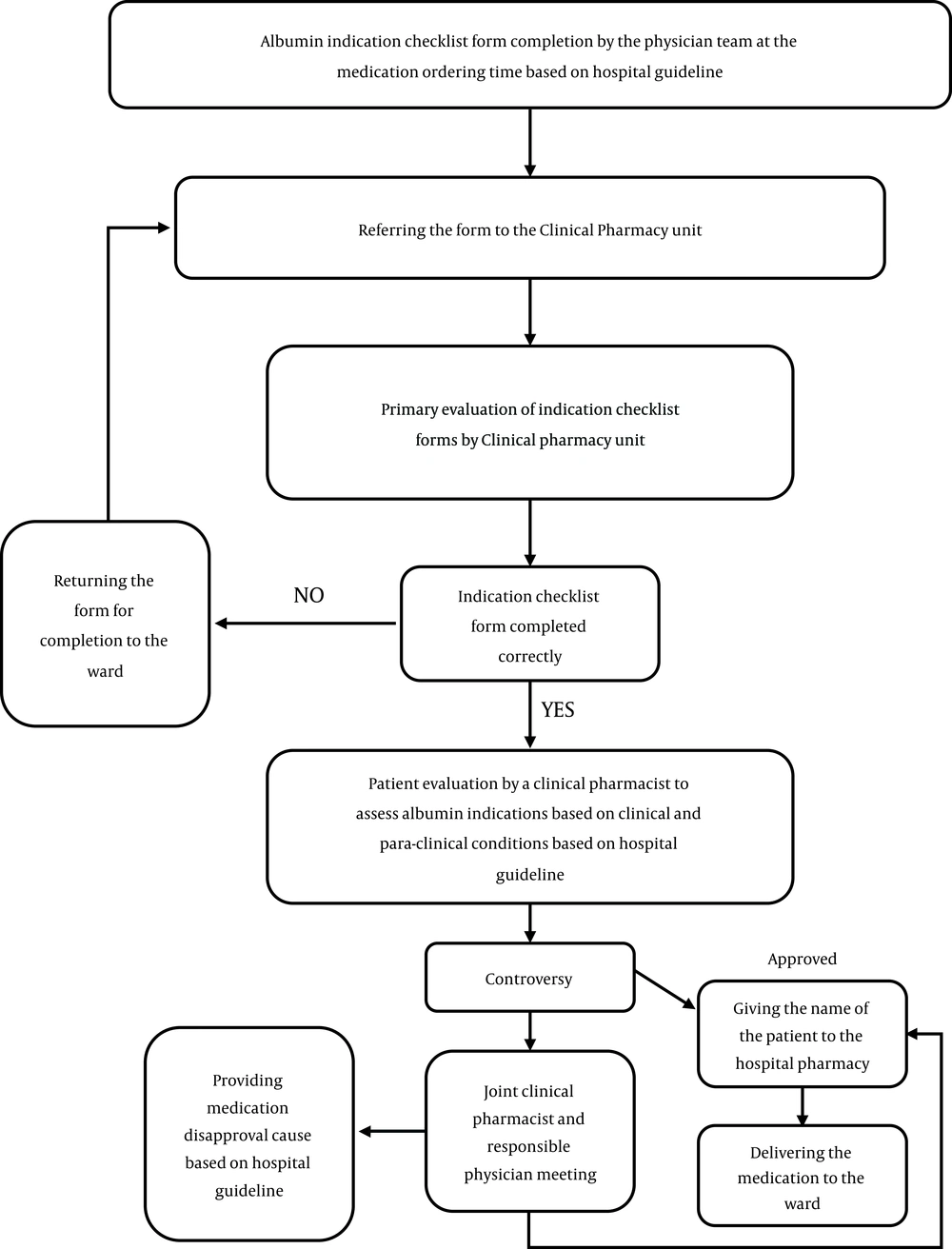
Thereafter, in the post-intervention phase, after coordination with hospital departments, after albumin request by the physician order, the request was examined based on the guidelines by a clinical pharmacist according to clinical and paraclinical findings. Clinical pharmacist was authorized to consult on approval or disapproval of the request. The physicians did receive educational courses on appropriate use of albumin in pre- or post-intervention phases by their request in a non-mandatory way. After the clinical pharmacist’s visit, a list of approved or disapproved patients, who were requested to use albumin for, was given to both the ward and hospital pharmacy. Figure 1 illustrates the flow chart of the clinical pharmacy unit guidelines.
3.4. Data Collection
Data collection was performed by a pharmacist under supervision of a clinical pharmacist and consisted of a checklist with the following items: (1) patient’s information (including demographics such as age, sex, height and weight, admitted ward, admission time); (2) patient’s nutritional status (including Nutritional Risk Screening (NRS) score, type of nutrition, times and amount of food; (3) albumin indication; (4) albumin order; (5) administration date and therapy duration; (6) clinical and para-clinical data (including albumin serum level and total protein, liver function test, complete blood counts, renal function tests, electrolytes and biochemistry, blood pressure and body temperature; (7) patient’s medication history; and (8) final decision.
3.5. Statistical Analysis
Statistical analyses were conducted as descriptive and analytical. Categorical variables were expressed as percentage. Chi-square or Fisher exact (if 20% of the variables have frequency of less than 5) were performed to analyze the correlation between these variables. Kolmogorov-Smirnov test was performed to examine the normal distribution of continuous data. Normally and non-normally distributed continuous variables were expressed as mean ± standard deviation (SD) and median (interquartile range), respectively. A comparison between parametric and non-parametric continuous variables was performed by independent t-test and Mann-Whitney, respectively. P value less than 0.05 was considered statistically significant in all analyses. All the above analyses were conducted by the Statistical Package for the Social Sciences (SPSS) version 21 software (IBM Company, New York, NY, United States).
4. Results and Discussion
In pre-intervention and post-intervention phases, 72 and 28 patients were recruited to gather albumin prescription data, respectively. In addition, 81% and 19% of the patients were recruited from intensive care units (Neurosurgery ICU, General ICU and Emergency ICU) and internal ward, respectively. There were no significant differences regarding number of patients in different wards in pre- and post-intervention phases (P = 0.508). Albumin were prescribed mostly by internists in pre-intervention (33.34%) and post-intervention (46.42%) phase of the study. The mean ± SD age of the patients in pre- and post-intervention phases were 57 ± 18.12 and 56.03 ± 17.81 years, respectively with no statistically differences between two groups (P = 0.800). Furthermore, no differences were observed in sex distribution between the two groups (P = 0.487).
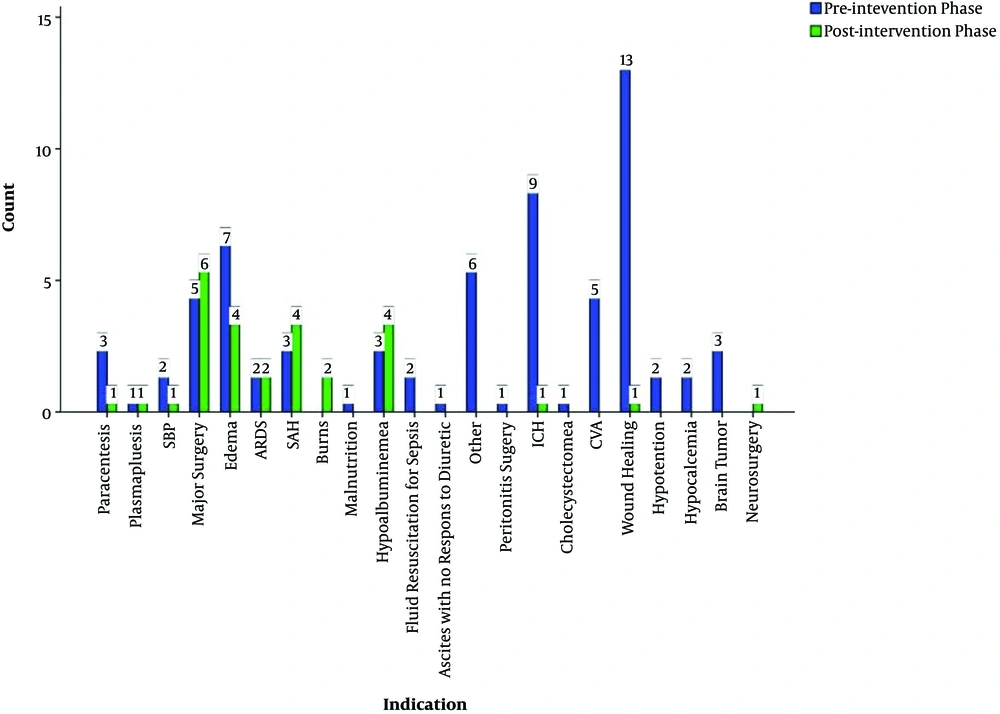
In the pre-intervention phase, the 3 most frequent indications were as follows: Wound healing (18.05%), Intracerebral hemorrhage (12.5%), and Edema (9.72%) and in the post-intervention phase: Major surgery (42.21%), Edema (14.28%), Subarachnoid hemorrhage (14.28%). Figure 2 presents all albumin indications in two phases. Data showed that albumin indication in post-intervention was significantly different from that in the pre-intervention phase (P = 0.043). Only 13 out of 72 indications were correctly identified based on the hospital guidelines in the pre-intervention phase. However, 18 out of 28 indications in the post-intervention phase were correct. The number of correct indications was statistically different between the two groups (P < 0.001). Regarding type of nutrition (Enteral nutrition, Nil Per OS or Per Os), frequency of physician specialty prescribing the medication, type of received intravenous fluid, receiving vasopressors and receiving inotropes, we did not identify statistically significant differences in pre- and post-intervention phases (P = 0.228, P = 0.531, P = 0.052, P = 0.985, and P = 0.256, respectively). Twenty nine percent of the patients received furosemide to treat edema in the pre-intervention phase, and 35% of the patients received this agent in the post-intervention phase. Comparing the number of patients and the dose of furosemide between the two groups, we did not identify a statistically significant difference (P = 0.452, P = 0.357, respectively). The mean furosemide dose in pre-intervention phase was 40.66 ± 49.26 and in post-intervention phase was 37.30 ± 16.04 mg per day. The patients in the post-intervention phase received albumin with a period of 5.5 ± 5.26 days, which compared to the pre-intervention phase, a period of 8.15 ± 7.72 days was shorter (P = 0.049). About one-third (32.62%) of total used vials were with correct indication in the post-intervention phase. On the contrary, in the pre-intervention phase, 6.28% were of total used albumin vials with correct indication. In the pre-intervention phase, 444 albumin vials and in post-intervention phase 249 albumin vials were consumed.
In comparison of albumin serum level before albumin transfusion, in pre-intervention phase higher levels were detected versus post-transplant phase (P = 0.028). Post transfusion albumin levels did not differ statistically significant in pre- and post-intervention phases (P = 0.058). Tables 1 and 2 show the important laboratory data of pre- and post-intervention phases and their distribution between two the groups. Apart from serum potassium (P = 0.022), total protein (P = 0.008), and initial albumin level (P = 0.028), other laboratory parameters were comparable between pre- and post-intervention periods.
| Laboratory Parameter | Pre-Intervention | Post-Intervention | P Value |
|---|---|---|---|
| White blood cells (× 103 cells/mm3) | 12.60 ± 8.26 | 13.75 ± 7.67 | 0.388 |
| Hemoglobin (g/dL) | 22.18 ± 6.39 | 10.30 ± 2.03 | 0.833 |
| Blood urea nitrogen (mg/dL) | 31.98 ± 23.86 | 37.29 ± 27.83 | 0.397 |
| Serum creatinine (mg/dL) | 1.29 ± 0.99 | 1.41 ± 0.92 | 0.564 |
| Blood sugar (mg/dL) | 129.91 ± 45.07 | 138.51 ± 60.65 | 0.602 |
| Sodium (mEq/L) | 139.16 ± 5.68 | 137.82 ± 5.28 | 0.282 |
| Potassium (mEq/L) | 3.88 ± 0.62 | 4.20 ± 0.60 | 0.022 |
| Calcium (mg/dL) | 8.03 ± 1.14 | 8.15 ± 1.07 | 0.646 |
| Alanine aminotransferase (Units/L) | 59.38 ± 91.38 | 43.50 ± 36.95 | 0.362 |
| Aspartate aminotransferase (Units/L) | 63.11 ± 84.59 | 46.33 ± 22.55 | 0.772 |
| International normalized ratio | 1.52 ± 0.68 | 1.47 ± 0.51 | 0.986 |
| Partial thromboplastin time (sec) | 34.37 ± 8.50 | 36.31 ± 10.35 | 0.341 |
Pre- and Post-Intervention Phases Laboratory Data and Their Distribution Between Two Groupsa
| Laboratory Parameter | Pre-Intervention | Post-Intervention | P Value |
|---|---|---|---|
| Total protein concentration | 5.35 ± 0.84 | 4.82 ± 0.88 | 0.008 |
| Initial albumin level | 2.94 ± 0.63 | 2.62 ± 0.45 | 0.028 |
| End of the study albumin level | 3.48 ± 1.06 | 3.07 ± 0.58 | 0.058 |
Total Protein and Albumin Serum Concentration in Pre- and Post-Intervention Phasesa
Drug utilization program and guidelines implementation are proven tools to obtain standards in medication use in hospitals. Interventional strategies employed by the World Health Organization in promoting rational drug use as well as medication management in hospitals, including educating and administrative actions, provide better access to limited source medications and offer clinical and economic benefits to healthcare systems (11). In this study, in a teaching hospital over 9 months, we investigated clinical pharmacist’s intervention in albumin rational use as high-cost medication with limited resources. In the post-intervention phase, after clinical pharmacist intervention, we observed that albumin consumption and less missuses based on incorrect indications reduced.
In the past decades, in different healthcare systems, inappropriate albumin prescriptions was reported, which was responsible for albumin irrational utilization at least in 50% of prescriptions in different countries (12-14). Owing to high cost and limited resources, albumin overuse could be a challenge to healthcare systems (12). It is indicated by the Iranian Food and Drug Organization in a 9-month period in 2008, 472,089 vials of albumin 20% were consumed, which amounted to approximately 21 million USD (15). Albumin usage also causes some serious concerns due to adverse effects. Severe anaphylactic reactions, coagulation abnormalities and electrolyte disturbances were reported with its usage. Some of these adverse reactions are caused by large replacement of volumes and need patients monitoring, and some of them occurs rapidly and need immediate discontinuation. Furthermore, albumin should be used with caution in conditions which hypervolemia and hemodilution may increase the risk of adverse effects such as heart failure, hypertension, and pulmonary edema (16, 17). Most of the albumin vials in our study were prescribed by internists. It was noted in previous studies that, albumin prescription not based on guidelines could lead to false prescription (14). It was also stated before that we could decrease patients and health system costs by limiting albumin prescription (18-20). Clinical pharmacist interventions and guidelines implementation in our study lead to 46.23% reduction in albumin use, and it was close to previous data (19). In line with our data, albumin guidelines implementation in an ICU in a teaching hospital in the Unites States resulted in statistically significant reduction of albumin use (54%) and lower costs (56%) (21). In a study by Miguel et al., to determine the impact of education on albumin irrational use, after providing a set of guidelines on albumin indications, physicians could prescribe albumin only based on the guidelines and they received education about albumin rational use, which was provided by a clinical pharmacist. The study showed a 37.2% reduction in wrong prescription and 30% reduction in cost after education (22). In our study, we observed a reduction in wrong prescription after providing the albumin use guidelines, but we could not determine the financial impact.
In our study, in the pre-intervention phase, we observed that the three most frequent indications for albumin prescription were as follows: (1) wound healing, (2) ICH, (3) edema, and only 18.05% of prescriptions were correct based on the guidelines. However, after providing the guidelines, 64.28% of the prescriptions were correct. In the pre-intervention phase, we observed that patients received furosemide by a mean dose of 40.66 ± 49.26 mg, which was lower than doses we could use to treat edema, and this could lead to increase albumin utilization to treat refractory edema. Roberts et al. compared albumin to treat hypovolemia with low-cost fluid such as crystalloids in critically ill hospitalized patients with hypoalbuminemia. This study showed no evidence of reduced morbidity and mortality in burnt patients with hypoalbuminemia. Regarding albumin price, this study indicated that albumin should be used only for absolute proven scientific indications (23). This issue was also studied before in patients hospitalized in ICU, which albumin and normal saline for fluid resuscitation resulted in similar outcomes in 28 days (24). Based on previous data, the use of albumin in severe sepsis and septic shock patients can reduce mortality and morbidity rate versus crystalloids (25, 26). Comparison between albumin and crystalloids was not in the scope of our study, but based on the medication cost and clinical and financial benefits, it would be necessary to evaluate this parameter in our hospital.
In our study, in the pre-intervention phase, most physicians defined hypoalbuminemia as serum albumin concentration of 3 g/dL or less, but in most of the studies, it was shown that albumin would be beneficial if serum albumin was less than 2.5 g/dL. In our study, we defined hypoalbuminemia as serum albumin less than 2.5 g/dL, and patients with hypoalbuminemia received albumin to correct serum albumin for 2.5 g/dL or more (5). In our guidelines, we consider five absolute indications for albumin usage as follows: (1) large volume (more than 4 liter) of therapeutic paracentesis in patients with ascites; (2) plasmapheresis as a replacement of plasma; (3) spontaneous bacterial peritonitis in patients with cirrhosis; (4) diagnosis of suspected hepato-renal syndrome; (5) treatment of confirmed hepato-renal syndrome. In these five indications, albumin showed its efficacy. However, in some indications such as nutritional interventions in patients with serum albumin above 2.5 g/dL for wound healing or in burnt patients in the first 24 hours, albumin is not indicated and should not be used. In the guidelines, we provided indications, which albumin may be beneficial. For example, in patients with subarachnoid hemorrhage, albumin is part of triple H therapy, and it was previously shown that albumin would be beneficial in these patients. In patients with symptomatic vasospasm, after securing aneurysm the triple H therapy which is involved the induction of Hypervolemia, Hypertension and hemodilution may be considered. In this regimen the albumin is indicated if the serum albumin level is less than 3 g/dL and the aneurism is secured. The goal of central venous pressure is 6 - 8 cm H and 8 - 12 cm H, if delayed vasospasm occurred. We indicated that albumin would be used in these patients if serum albumin is less than 3 g/dL (27-30). We also considered 17 more other indications, which albumin may be beneficial. As we observed in our study, guidelines implementation in human albumin use in our hospital changed prescription pattern and reduced wrong prescriptions. In a 3-phase study (observation, guidelines provision and instruction to use) in 2017, albumin pattern use in hospital was assessed. In this study, similar to our study, it was showed that, after providing the albumin indication guidelines, consumption pattern changed and wrong prescription decreased (31). Our data may not be generalizable to all hospitals in Iran, and we need to investigate similar studies in different centers to provide general DUE program for albumin use in Iran; however, this may lead to increase albumin rational use and decrease costs of the healthcare system, which irrational drug utilization imposes additional costs on the healthcare system.
There were some limitations in our study: (1) small sample size; (2) single centered study design; (3) in holidays and night shifts due to absence of a clinical pharmacist, some patients received albumin without consultation; (4) not including financial benefit of albumin utilization program; and (5) in the study duration, to obey the guideline was not mandatory and in some cases the medication could be delivered to wards without clinical pharmacist approval.
5.1. Conclusions
In conclusion, our study demonstrated that in Loghman-Hakim Hospital, albumin utilization was irrational and was not based on correct indications; therefore, we reduced irrational prescriptions by providing and using guidelines as well as correcting the prescriptions under supervision of a clinical pharmacist. The use of proper albumin indication guidelines in the medical center based on documented and scientific indication will improve drug utilization program along with cost saving.