1. Background
Nowadays, due to the numerous side effects of conventional and chemical drugs, it is necessary to find new alternative therapeutic drugs for many diseases (1). Human beings have used plants in traditional medicine for ages due to their therapeutic effects. The search for medicinal plants has led to the identification of novel drug candidates for the treatment of different diseases (2). According to the research on plants rich in natural antioxidants, Pistacia atlantica (PA) is one of the useful plants with antioxidant therapeutic properties. Pistacia atlantica is a pistachio species and belongs to the Anacardiaceae family, which includes 13 species. There are more populations of Pistacia in the Middle East, Northern Africa, and the Mediterranean region of Europe compared to other parts of the world (3-5).
Some species of Pistachio, including Pistacia vera L., Pistacia khinjuk Stocks, and PA, can be found in Iran (3, 4). Pistacia atlantica, which is vernacularly called Bene in Persian, is a tree rich in phenolic compounds, including flavonoids, phenolic acids, and free radicals (e.g., hydrogen peroxide, lipid hydroperoxide, and peroxyl radical) (6). Different parts of PA, including its fruit, leaves, and oil, have been reported to have several therapeutic applications. Natural products, such as oleoresins, terpenoids, catechins, and flavonoids, can be used as alternative therapies (7, 8). Pistacia atlantica fruit and leaves extracts are a natural source for the production of new scolicidal agents and contain monoterpene hydrocarbons, including β-myrcene (about 40%), α-pinene (about 32%), and limonene (8). Moreover, PA leaves are characterized by phenolic composition, flavonoids, and condensed tannins contents, which are the main sources of antioxidant activity in PA leaves (9) Pistacia atlantica leaves extract has been used as a therapeutic agent in the treatment of gum diseases, wounds, throat infections, stress, stomach disorders, and diarrhea (10, 11). Moreover, some Pistacia species evaluated for their protective effects against some complications, such as hyperuricemia and gout, have been reported to have anti-inflammatory and analgesic properties (12, 13).
Since few studies have investigated the analgesic effects of ethanolic extracts from the gum, leaves, and fruit of Pistacia species (7, 14), the current study aimed to examine and compare the analgesic activities of different parts of PA in rats treated with different concentrations of the hydroalcoholic extract of PA. The analgesic effects were assessed by the tail-flick and formalin tests. Although no phytochemical analysis has been carried out on PA so far, the fruits of the plants belonging to the Anacardiaceae family are known to be a source of structurally diverse flavonoids and terpenes, which are capable of having analgesic properties unique to this family of plants.
2. Objectives
This study was designed to evaluate the analgesic activity of the hydroalcoholic extract of PA leaves, gum, and fruit in rats.
3. Methods
3.1. The Preparation of the Hydroalcoholic Extract of PA Leaves, Gum, and Fruit
Different parts of PA, including leaves, gum, and fruit, were acquired from a shop in Shiraz, Fars Province, south of Iran and authenticated by the taxonomists of School of Pharmacy, Shiraz University of Medical Sciences (SUMS), Iran, in March 2019 (fruit Pm-494, leaves Pm-495, gum Pm-496). To prepare the decoction, the fruit and leaves were first cleaned and then shaded for four days. Afterward, 700 gr of the plant powder was extracted with an ethanol-water mixture (80:20) at room temperature overnight. A rotary evaporator was used to completely remove the solvent at 50°C. Finally, the extracts were freeze-dried and kept at - 50°C until further use. The gum was extracted from the resin by cold maceration and hydrodistillation with ethanol (1:5). The combined hydroalcoholic extract was filtered through a filter paper and evaporated at 50°C. The gum extract was kept in dark containers and refrigerated until testing (7).
3.2. The Determination of Total Phenolic Content (TPC)
The reduction of Folin-Ciocalteu reagent by phenolic compounds was used to determine the TPC of the hydroalcoholic extract of PA leaves. Stock solutions of leaves extract at different concentrations were prepared in MeOH. First, 200 μL of Folin-Ciocalteu reagent was mixed with 40 μL of the extract sample; then, 3.2 mL of water was added. After around 10 min of incubation at room temperature, 600 μL of 0.25% sodium carbonate was added to the solution. The prepared solution was incubated for an additional 2 h at 37°C in the dark. The same procedure was repeated for gallic acid as the standard solution (15, 16). The calibration curve was plotted using gallic acid. The optical density was measured at 765 nm using a Bio-Rad microplate reader (Model 680, Bio-Rad). The concentration of TPC was expressed as mg of gallic acid equivalents per g of dry extract.
3.3. Antioxidant Assay
The antioxidant potential of the hydroalcoholic extract of PA leaves was determined using the Brand-Williams DPPH assay. Test solutions (270 μL), containing different concentrations of the plant extracts in methanol were mixed with 20 μL of a methanolic solution of DPPH (100 μM). This mixture was incubated at room temperature for 30 min. Then, absorbance was measured at 517 nm by a spectrophotometer. Each experiment was repeated three to five times (17, 18). Methanol was used as the blank for the microplate reader. The results of DPPH assay were expressed as IC50 value using Curve-Expert software (version 1.34).
3.4. Selection of Animals
Seventy-two adult male Sprague-Dawley rats (220 ± 20 g, 10 - 12 weeks) were collected from the laboratory animal center of Shiraz University of Medical Sciences (SUMS), Iran. The animals were randomly divided into 12 groups and maintained in a standard condition, with controlled temperature (22 ± 2°C) and 12-hour light and dark cycles. They had free access to food and water ad libitum. The ethical guidelines approved by the Ethics Committee of SUMS regarding the protection of animals used for experimental purposes were followed in all tests.
3.5. Experimental Design
After a 7-day adjustment period, the rats were randomly divided into 12 groups, each containing six rats, as follows: Group I: Control group, normal healthy rats; Group II: Negative control group, rats received vehicle ( Dimethyl sulfoxides (DMSO(, 250 μL/kg, IP); Group III: Positive control group, rats received the standard drug (APAP 100 mg/kg, IP); Groups IV-VI): Rats received 25, 50, and 100 mg/kg of the leaves extract(IP); Groups VII-IX): Rats received 25, 50, and 100 mg/kg of the gum extract (IP); and Groups X-XII: Rats received 25, 50, and 100 mg/kg of the fruit extract (IP).
3.6. Analgesic Tests
In this study, two types of painful stimuli (thermal and chemical) were used to induce pain in rats by direct stimulation of pain receptors. The tail-flick and formalin tests were used to measure responses to thermally- and chemically-induced pain, respectively. For each test, the rats were used individually.
3.7. Tail-flick Test
A tail-flick analgesia meter (Borj Sanat, Iran) was used to measure the pain threshold. The light intensity was adjusted to 70% of the maximum. A 15-second cut-off was imposed to avoid tail damage. The tail-flick latency time was measured in seconds. The positive control group received an IP injection of 100 mg/kg of paracetamol. The negative control group received 250 μL/kg of DMSO, IP. The animals in groups IV-XII received 25, 50, and 100 mg/kg of the hydroalcoholic extract of PA leaves, gum, and fruit, respectively. Analgesic activity was assessed by recording the reaction time. Following the administration of vehicle, paracetamol, and extracts, the reaction time was recorded at 30 and 60 min.
3.8. Formalin Test
To induce tonic pain in rats, 30 minutes after the injection of vehicle, paracetamol, and treatment extracts, 50 μL of 2.5% formalin (37% formaldehyde) in distilled water was injected subcutaneously into the right hind paw of the rats. It should be noted that the injectable solution did not penetrate deep into the tissue. Another important point to take into consideration is that intradermal injection cannot produce a long-lasting response. Analgesia was assessed using the formalin method (19). Pain behavior was scored as follows: 0 = the injected paw was not favored; 1 = the injected paw had little or no weight placed on it; 2 = the injected paw was elevated and not in contact with any surface; and 3 = the injected paw is licked or bitten. The animals’ responses were monitored for 1 h after formalin injection. The first phase was 5 minutes, and the second phase was 20 - 60 minutes after the formalin injection (19)
3.9. Statistical Analysis
Data was presented as mean ± standard deviation (SD). The data was analyzed using one-way analysis of variance (ANOVA) with Dunnett’s post hoc test. A P-value of less than 0.05 was considered statistically significant.
4. Results
The TPC in the hydroalcoholic extract of PA leaves was calculated using the calibration curve, and the results showed that the hydroalcoholic extract of PA leaves had a TPC value of 433.28± 9.46 as mg GAE/g dry extract (1). The IC 50 value for DPPH radical scavenging activity was found to be 1.57 ± 0.28 mg/mL. Quercetin was used as a reference antioxidant (IC 50 = 9.1 ± 0.42 μM), and the data was presented as mean ± S.D.
4.1. The Analgesic Effects of Leaves, Gum, and Fruit Extracts Based on the Tail-flick Test
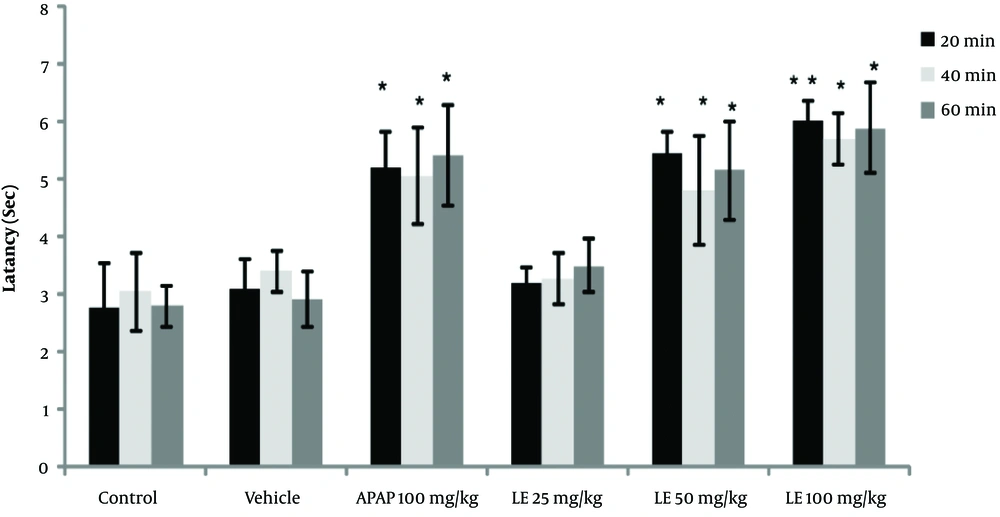
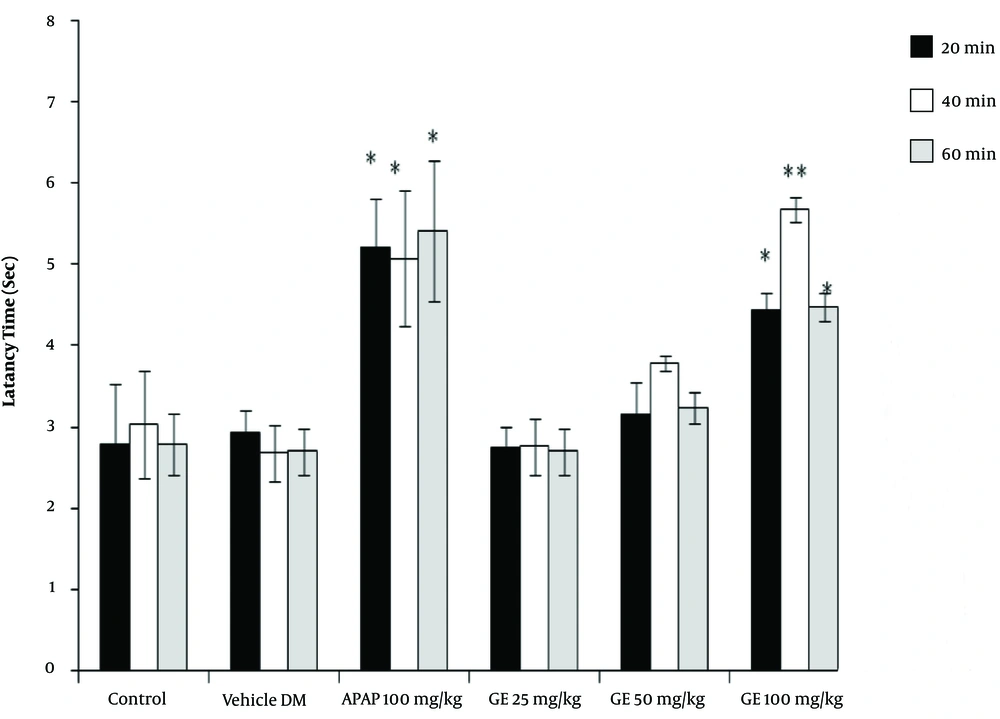
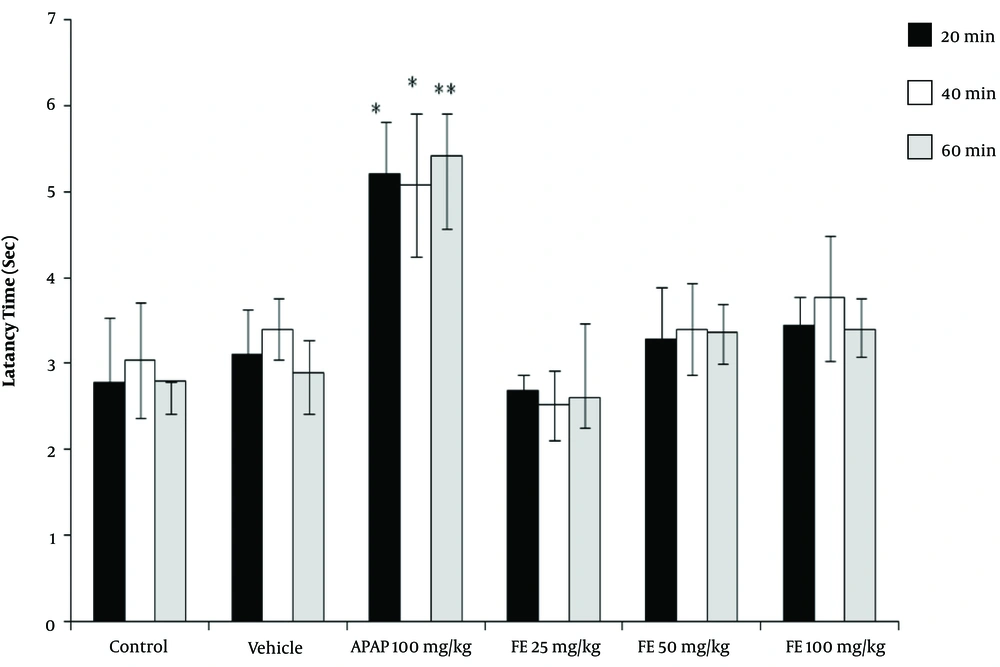
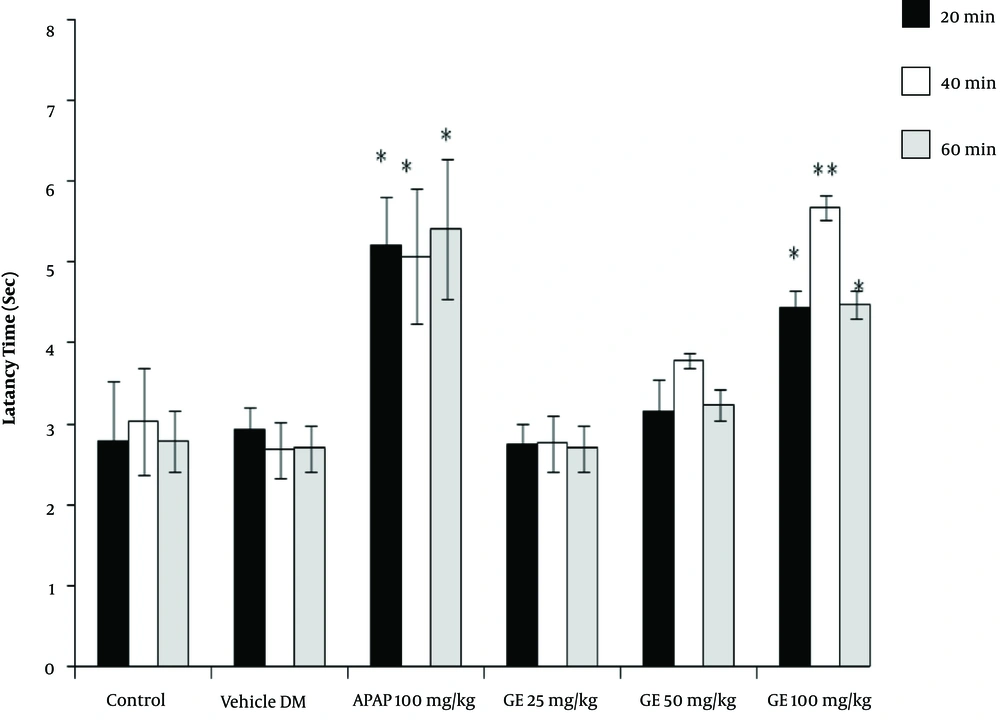
The results of the tail-flick test showed that the high concentrations of PA leave extract (50 and 100 mg/kg) had analgesic properties, as revealed by the increase in the latency time compared with the control groups (P < 0.05). However, the leaves extract did not have any significant effect at a dose of 25 mg/kg (Figure 1). Moreover, the PA gum extract had analgesic effects at the doses of 100 and 200 mg/kg, as evidenced by the increase in the latency time compared with those of the control groups. However, the PA gum extract at the low doses of 25 and 50 mg/kg had no significant analgesic effect (Figure 2). The results also revealed that the PA fruit extract had no significant analgesic effect at any of the three doses because no significant difference was observed in the latency time compared with those of the control groups (Figure 3).
4.2. The Analgesic Effects of Leaves, Gum, and Fruit Extracts Based on the Formalin Test
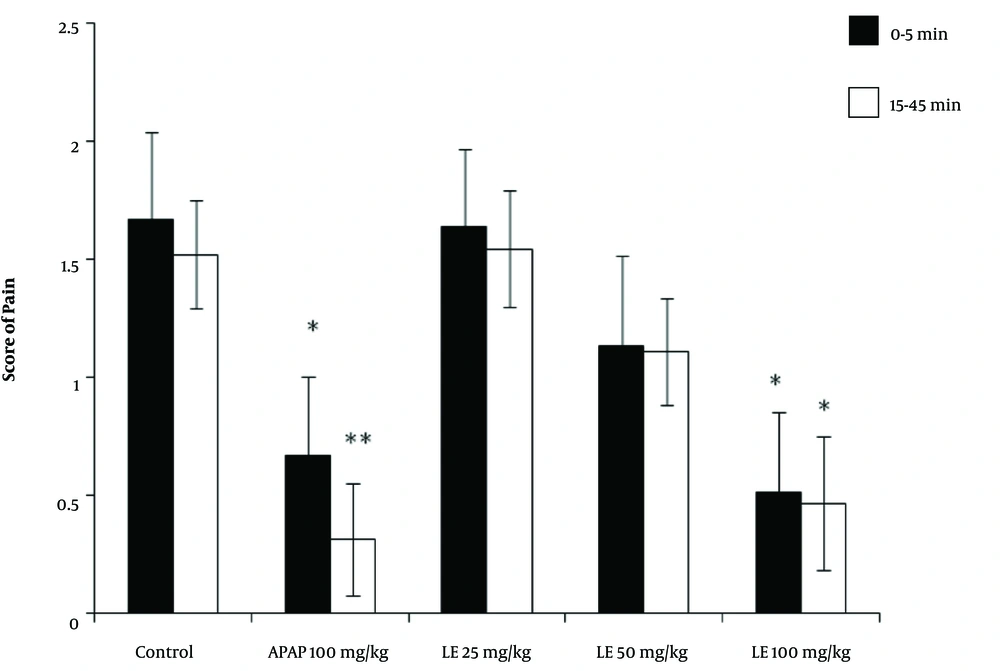
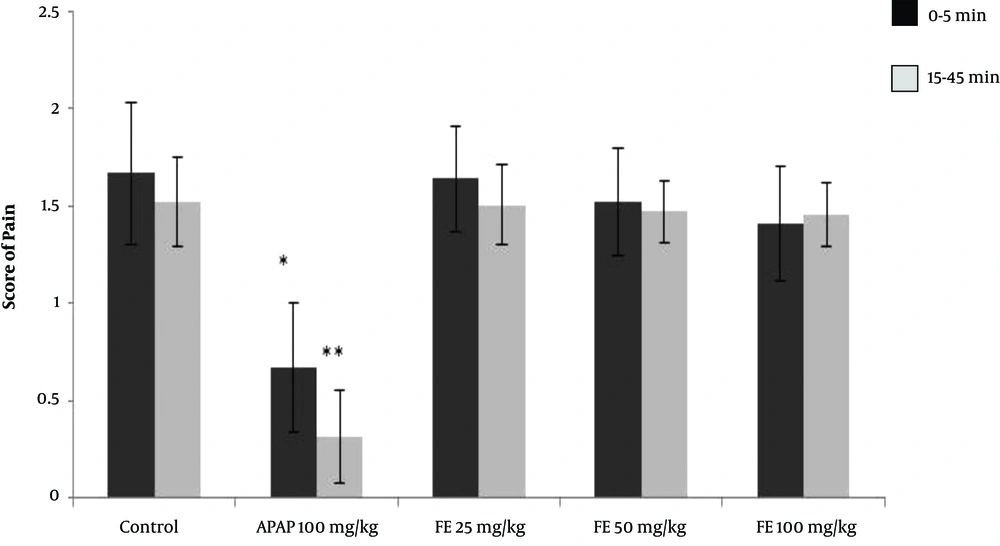
Following the injection of formalin in the control group, the average pain score reached its highest level during the first phase (first 5 minutes) and then decreased gradually during the second phase (15 to 45 minutes). The pain score between the two phases (5 to 15 minutes) is not usually reported because it does not affect the test results. The rats receiving paracetamol experienced a significant decrease in pain score in the first 5 minutes. The reduction in the pain score was even greater in the second phase, with pain levels below 0.5. The rats receiving 100 mg/kg of the leaves extract experienced a significant reduction in pain score in both the first and second phases compared with that of the control group. (P < 0.05) However, in rats receiving 50 mg/kg of leaves extract, the reduction in pain score in the first and second phases was not significantly different from that of the control group. The rats receiving 25 mg/kg of the leaves extract showed neither analgesic effect nor any reduction in pain in either the first or the second phase of the test (Figure 4).
The results of the formalin test and the pain score in different animal groups treated with the hydroalcoholic extract of PA leaves. Data is given as Mean ± SD. APAP: Paracetamol, LE: Leave extract. The asterisk (*) indicates significantly lower scores compared with that of the control group (P < 0.05).
The gum extract at a dose of 100 mg/kg alleviated pain in both phases of the formalin test and had a stronger analgesic effect than did paracetamol. The difference was significant when the results were compared with that of the control group (P < 0.05). Our results showed that the gum extract had no significant effect at the doses of 25 and 50 mg/kg (Figure 5). No analgesic effect was observed at any of the doses of the fruit extract in either of the phases (Figure 6).
5. Discussion
Based on the results of this study, different concentrations of PA leaves extract, especially at a dose of 100 mg/kg, could alleviate pain more effectively than could the PA gum and fruit extracts in the formalin and tail-flick tests in rats. This could be due to the higher concentration of components with analgesic properties in the leaves extract compared with those in the gum and/or fruit extracts. The PA fruit extract showed no significant analgesic activity in the current investigation.
Pain is described as an increased sensitivity of the injured tissue, which happens when some inflammatory mediators are released (20). The pharmacological treatment of pain usually includes nonsteroidal anti-inflammatory drugs (NSAIDs), which have been reported to be highly effective (21). Nowadays, researchers are looking for other pharmacological strategies, such as natural plants (9). Monoterpenes are extracted from several herbs and constitute 90% of essential oils. Regarding the pharmacological activities of monoterpenes, about 30 monoterpenes have been found to have analgesic and anti-inflammatory activities (22, 23). Former studies on the therapeutic effects of Pistacia species have revealed that some species, such as Pistacia vera L. and Pistacia integerrima, have anti-inflammatory and antinociceptive activities, which is in line with the results of the present study (17, 21) Some studies have attempted to investigate the chemical composition of some constituents, such as fatty acids, sterols, terpenes, α-tocopherol, and flavonoids, identified in different Pistacia species and have reported that Pistacia species are a natural source of new scolicidal agents (9).
It has also been documented that some flavonoids, terpenes, and sterols derived from natural plants have analgesic properties (24). Anti-inflammatory and antinociceptive effects of flavonoids are induced by the inhibition of IL-1β, IL-6, and TNF-α, as pro-inflammatory cytokines, and the suppression of the production of cyclooxygenase-2, leading to a reduction in pain reaction time (25). As low-molecular-weight compounds with high lipid solubility, terpenes can penetrate the blood-brain barrier and act in the central nervous system to exert their pharmacologic effects (26). As the dominant constituent in terpenes, alpha-pinene has the most potent activity among the monoterpenes due to its role in managing neuropathic pain (27). Oleanolic acid, a triterpenoid, plays an essential role in reducing pain by inducing the release of endogenous opioids (28, 29). The analgesic effects of PA extracts might be due to the above-mentioned chemical compounds. These compounds might be present in the extracts evaluated in this study in varying concentrations. In this study, the PA fruit extract was found to be ineffective in alleviating pain in rats. However, a comparison of the extracts from different parts of PA revealed that the hydroalcoholic extract of PA leaves had the strongest analgesic activity. We could not find any previous studies on the analgesic effects of PA leaves to compare our results with them. Future investigations on different constituents present in different PA extracts will shed light on the mechanism of activity of this plant and the specific ingredients responsible for its analgesic effect.
5.1. Conclusions
The results of this study suggest that the stronger analgesic effect observed for the PA leaves extract, compared with those observed for the extracts of other parts of PA, might be due to the higher concentration of analgesic components in the PA leaves.