1. Background
Osteoarthritis (OA) of the knee is a progressive, degenerative, and irreversible disease in the joint structures (1). The mechanism includes the destruction of the extracellular matrix and reduction of the chondrocyte function mainly due to inflammation processes (2). Although a great number of surgical and non-surgical treatment strategies have been reported in several studies, no efficient approach has been reported to stop or reverse the progressive nature of this destructive joint disease, decrease pain, and improve joint function (3). The gold standard treatment for OA is total knee joint replacement, which has many complications like all other surgical procedures, including joint inflammation, leg shortening or lengthening, infection, and bleeding (4). Many studies have tried stem cell therapy and bioengineering to regenerate the knee hyaline cartilage in patients with OA (5, 6).
All joint tissues have some resident mesenchymal stem cells (MSCs) that are capable of differentiating into joint structures (7). In OA patients, these MSCs are decreased and lose their phenotypes and differentiation potential (8). Mesenchymal stem cells show better responses than other cell types, such as chondrocytes, due to more rapid proliferation, easier culture ex-vivo, better specialization to all joint tissues, and better results in generalized cartilage loss, specifically in OA (9). Anti-inflammatory, anti-apoptotic, and immune-modulatory characteristics of MSCs also make them superior in cell therapy (10). It is assumed that the best MSC source for osteo-chondrogenic purposes is synovial membrane-derived stem cells due to greater chondrogencity and chondrogenic phenotypes in contrast to other cells from the bone marrow or intra-patellar fat pad (11, 12).
Due to the low survival rate of intra-articular-injected MSCs at the site of the lesion, it is hypothesized that MSCs only work as building blocks and their paracrine effects play a particular role in influencing the host MSCs by increasing the survival, migration, and differentiation potential (13). Furthermore, it evokes osteo-chondrogenic gene expression, such as the PTH-like hormone, bone morphogenetic protein, Indian hedgehog protein, and collagen type 2 (14). The cytokines and growth factors that are secreted into the medium of MSCs (secreta) have similar effects on bioactive factors extracted from transplanted MSCs in the knee joint area (15). Despite all these potentials, a few animal model studies have been conducted to demonstrate the paracrine effect of synovial stem cells on the bone and cartilage regeneration in osteoarthritis.
2. Objectives
We designed an in-vivo study to evaluate the efficacy of intra-articular injection of autologous synovial membrane-derived MSCs with and without secreta in rats with induced knee OA by using radiological and histological evaluation methods.
3. Methods
This study was conducted at Shiraz University of Medical Sciences, Shiraz, Iran, from February to December 2019. Synovial tissue samples for the study were obtained from the inner side of the medial joint capsule of the knee joint of mature male rats. All samples were digested with collagenase type 1 (Sigma-Aldrich, USA) at 37°C. After one hour, digested cells were filtered through a 70-µm nylon filter to yield single-cell suspensions. All obtained cells were cultured in a complete medium of DMEM (Dulbecco’s Modified Eagle’s low glucose medium). Eventually, all cultures were incubated in 5% CO2 at a temperature of 37°C.
Synovial stem cells’ characterization was revealed by the International Society of Cell Therapy. Stem cells’ immune-phenotypes were evaluated by a flow cytometer (BD FA CSCalibur, flow cytometer, USA) using surface markers including CD90, CD44, CD34, and CD45. Moreover, multi-potentiality was assessed by in vitro osteogenic and adipogenic differentiation.
Synovial-derived stem cells were cultured in 150 mm tissue-culture dishes at 37°C with an atmosphere of 90% air and 10% CO2 and then got rich with DEMD, which had no protein, fat, or growth factors. When the cell culture became confluent, synovial cells were rinsed with PBS and the growth medium was replaced with DMEM. After 24 hours, the conditioned medium was collected and centrifuged twice, first at 500 × g for 10 min and then at 3,000 × g for 20 min, to remove cell debris. Finally, pre-cleaned secreta was concentrated to ~1.5 mL using Centriprep YM-3 centrifugal units (Millipore, Bedford, MA, USA).
One week after the adaptation of rats to the animal laboratory condition, all of them received two separate intra-articular injections through the patellar ligaments on days 0 and 3, as a booster, with 500 U of collagenase type 2 extracted from clostridium histolyticum medium (Sigma-Aldrich, St. Louis, MO, USA). Collagenase type 2 was dissolved in saline and filtered through a 0.22 µm membrane before injection. During the surgical procedure, rats were anesthetized with 10 mg/kg xylazine 2% (Alfasan, Netherlands) and 100 mg/kg ketamine 10% (Alfasan, Netherlands). After 10 weeks, radiological imaging was done to confirm osteoarthritis in the knee joints of rats.
Fifty adult male Sprague Dawley rats (200 ± 20 g body weight and 8 - 10 weeks of age) were used in this randomized animal trial. All animals were kept in standard cages with a 12-h light/dark cycle, 22 ± 2°C temperature, and 55 ± 5% relative humidity. All animals had free access to water and food ad libitum. The rats were divided into five groups randomly (n = 10).
The first group was the control group that did not receive any treatment. The second group received an intra-articular Hyalgan injection (0.1 cc with a concentration of 20 mg manufactured by Fidia Italy). The third group received secreta in the form of intra-articular injection. The fourth group received an intra-articular injection of synovial derived stem cells (5 × 106), and the last group received an intra-articular injection of secreta in combination with synovial-derived stem cells (5 × 106). Three months after treatment onset, all rats were sacrificed by 70% CO2, and then the samples were harvested. The study followed the internationally accredited guidelines with ethical approval from the Institutional Animal Care and Use Committee of Transgenic Research Center of Shiraz University of Medical Sciences (Shiraz, Iran). The registration number was 94-01-67-10171.
For pathological evaluations, first, a gross examination of joint surfaces was done on sacrificed rats. Then, the distal femora were detached. Buffered formaldehyde (10%) was used for tissue fixation. All specimens were embedded in paraffin, sectioned in thicknesses of 5 µm, and stained with hematoxylin and eosin. A blinded pathologist performed the evaluations according to the International Cartilage Repair Society (ICRS). The scoring system was according to the following parameters: Cartilage mineralization, matrix, cell population viability, cell distribution, subchondral bone, and surface (16). More severe damage was indicated with a lower score. The grades included normal (ICRS score = 12), good (IRCS score = 8 to 11), abnormal (ICRS score = 7 - 4), and poor (ICRS score = 1 to 3). All morphometric indices were documented with a digital camera system (Olympus Optical, Tokyo, Japan).
The X-ray images from knee joints were taken from the lateral aspects of the left knee using the same equipment (Axiom Multix M Radiographic Unit, SiemensTM, Germany). Osteoarthritis was assessed according to a grading system based on ICRS (17). Scoring subjects were according to radiological indices such as joint space narrowing, presence of osteophytes, subchondral bone sclerosis, and bone ends deformity. The scores included 0 (none), 1 (doubtful), 2 (minimal), 3 (moderate), and 4 (severe). Osteophytes in the medial condyle of tibia, femur, medial fabella, total knee joint, joint space width, and total OA score were evaluated by a blinded radiologist.
Data are expressed as mean and standard deviation (SD). Non-parametric tests such as Kruskal-Wallis (distribution-free) and Mann-Whitney U-tests were used for statistical comparisons of the histopathological and radiological grades between the groups. A P-value of < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS version 21.0 (IBMTM, USA).
4. Results
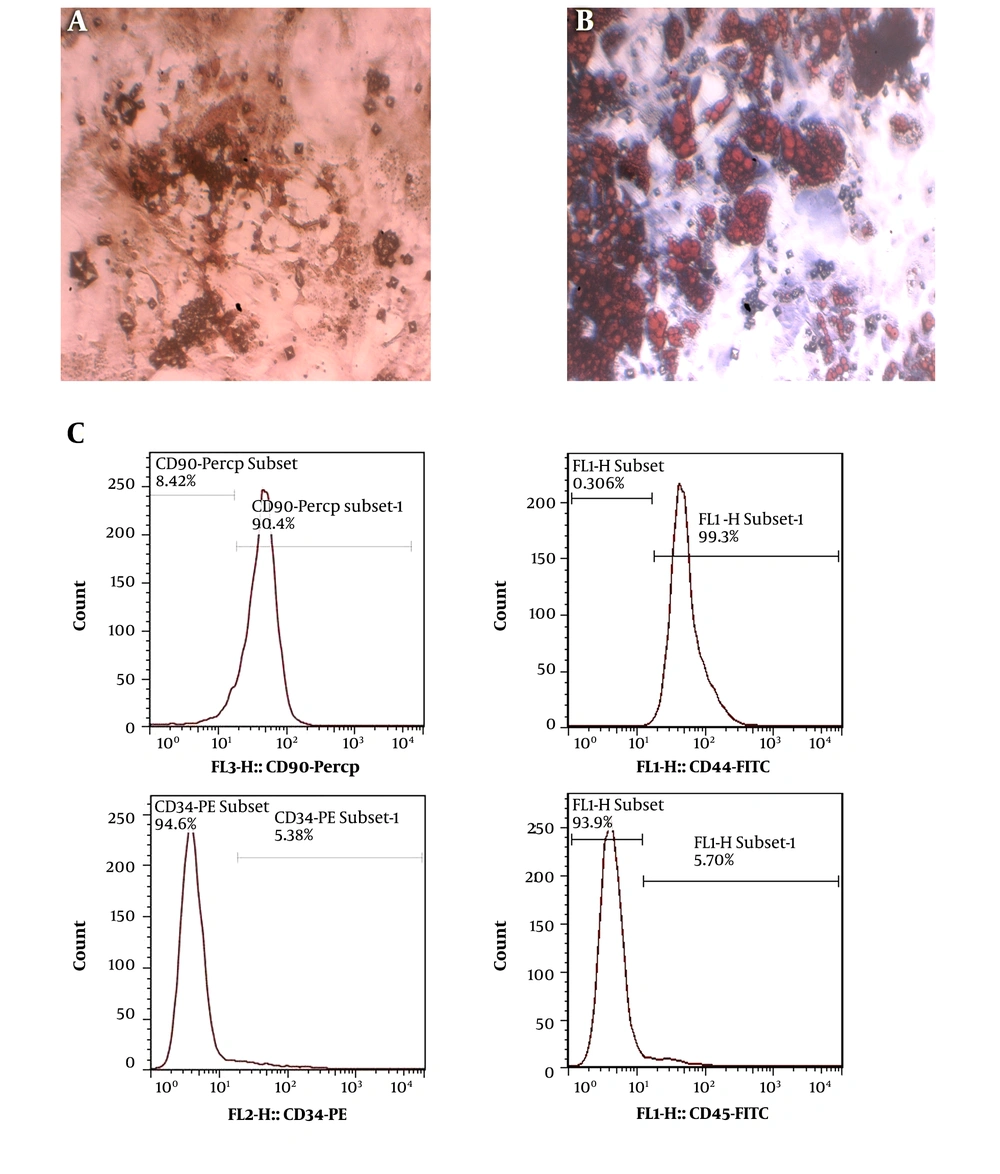
Osteogenic and lipogenic differentiation was evaluated after three weeks. In the osteogenic differentiation evaluation, the plate with synovial-derived stem cells showed mineral deposition. To investigate the extent of mineralization, we performed alizarin red staining. Lipid vacuoles were stained with oil red O stain to prove the lipogenic differentiation potential. The immune-phenotype, which was evaluated by flow cytometry, showed positive surface markers of CD90 and CD44 and negative markers of CD34 and CD45 (Figure 1).
Morphology of rat synovial stem cells at passage 3. A, Oil red O staining used to distinguish adipogenic differentiation with positive staining of cytoplasmic lipid droplets; B, Alizarin red staining applied to realize osteogenic differentiation by the appearance of mineralized nodular structures; C, stem cell characterization evaluated by flow cytometry in rat models of osteoarthritis. The immune-phenotype of rat's synovial stem cells at passage 3 was analyzed for CD90, CD44, CD34, and CD45 expressions.
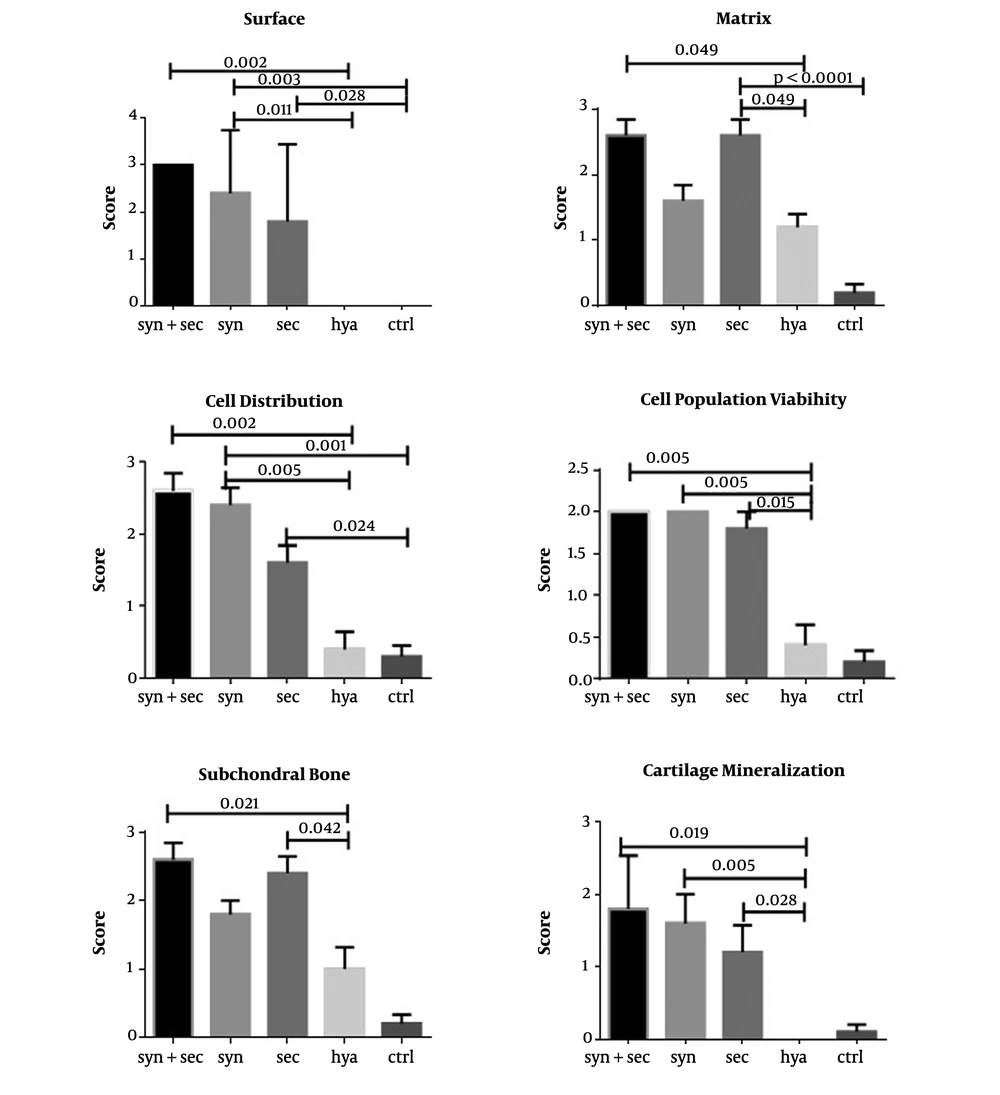
Histologically, 12 weeks after collagenase consumption, control knees exhibited an apparent defeat of cartilage in both lateral and medial condoyles, while all histopathological indices were predominantly retained in knees treated with synovial stem cells combined with secreta when compared to the Hyalgan and control groups. Statistical comparisons between the groups in all histological indices are shown in Figure 2.
The cell population viability index demonstrated better healing in both secreta and synovial-derived stem cell groups than in the Hyalgan group (P-values of 0.015 and 0.005, respectively) (Figure 2). The surface index demonstrated better healing in both secreta and synovial-derived stem cell groups than in the control group with P-values of 0.028 and 0.003, respectively. Moreover, the synovial-derived stem cell group showed a statistically significant difference from the Hyalgan group with a P-value of 0.011 (Figure 2).
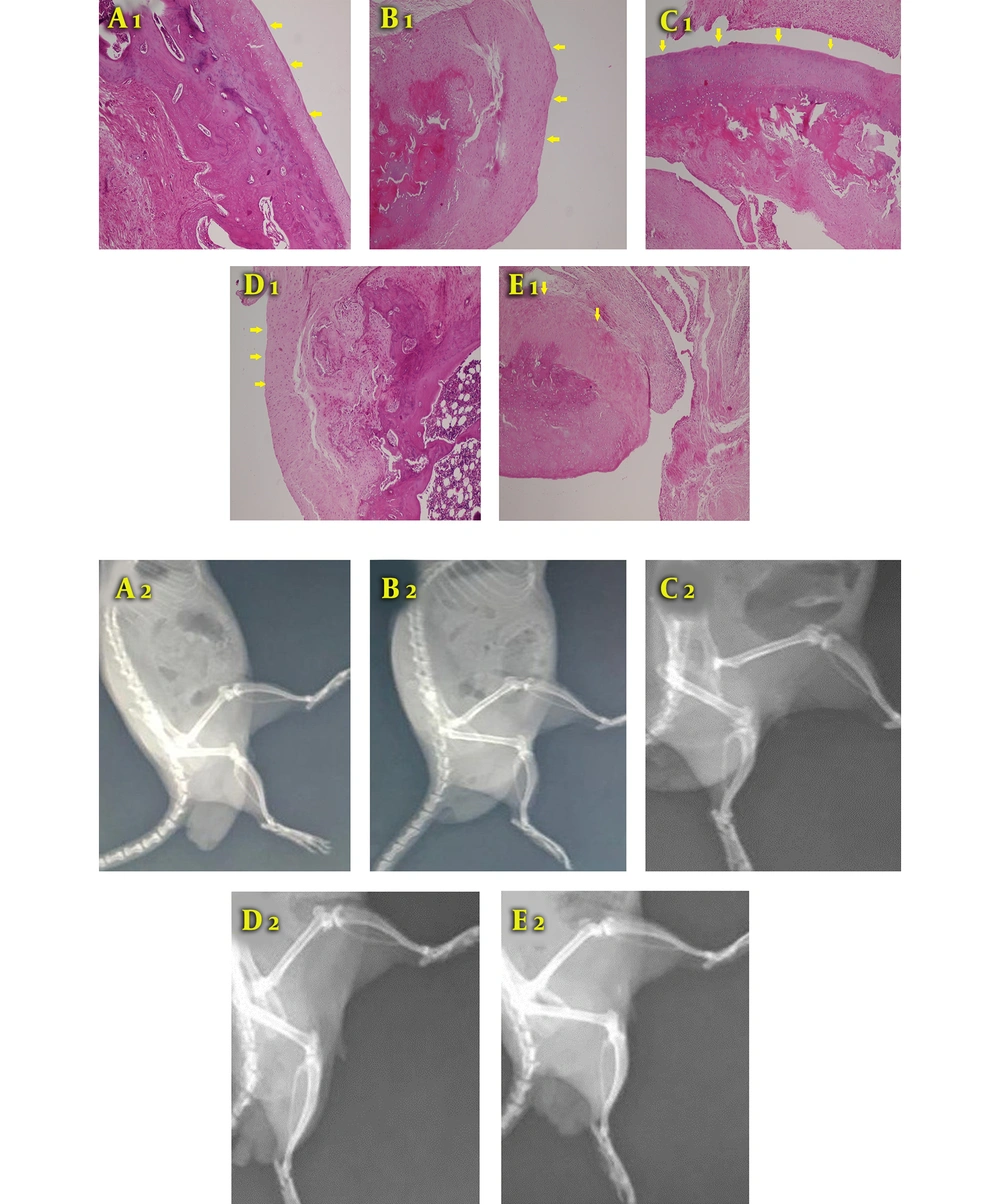
In the subchondral bone and matrix indices, the secreta showed better results when compared to the Hyalgan group with p-values of 0.042 and 0.0001, respectively (Figure 2). Besides, H & E staining showed that the group treated with secreta and synovial stem cells had articular hyaline cartilage with even and smooth surfaces along with arranged chondrocytes in the columnar cluster. Also, no foci of abnormal calcification were seen (Figure 3A).
Typical histological sections from the surface of articular cartilage in all groups (H & E, 200×). A1, Secreta with synovial stem cell group: Arranged chondrocyte in columnar-cluster along with the continuous and smooth surface of articular hyaline cartilage with no foci of abnormal calcification are observed; B1, Secreta group: The surface of articular cartilage was mainly continuous, composed of hyaline cartilage in a cluster arrangement. Subchondral bone remodeling was present; C1, stem cells group: The surface of articular cartilage was mainly continuous, composed of fibrocartilage tissue arranged in clusters. Subchondral bone was detached with increased remodeling; D1, Hyalgan group: The surface of articular cartilage was mainly continuous, composed of fibrocartilage with foci of hyaline cartilage cluster; E1, control group: The surface of articular cartilage was completely destructive and composed of disorganized fibrocartilage and fibrous tissues. Moreover, the subchondral bone was detached. Radiological images of knee joint 12 weeks after collagenase intra-articular injection. A wider joint space is seen in the synovial stem cells + secreta group than in the Hyalgan and control groups; A2, animal induced with collagen type 2 adjuvant and treated with secreta and synovial stem cells; B2, animal induced with collagen type 2 and treated with secreta; C2, animal induced with collagen type 2 and treated with synovial stem cells; D2, animal induced with collagen type 2 adjuvant and treated with Hyalgan; E2, animal induced with collagen type 2 as the control group.
In the secreta group, the surface of articular cartilage was mainly continuous, composed of hyaline cartilage in a cluster arrangement. Subchondral bone remodeling was present, as well (Figure 3B). In the stem cell group, the surface of articular cartilage was mainly continuous, composed of fibrocartilage tissue arranged in clusters. Moreover, the detached subchondral bone with increased remodeling was observed (Figure 3C). In the Hyalgan group, the surface of the articular cartilage was even mainly composed of fibrocartilage with foci of hyaline cartilage cluster (Figure 3D). In the control group, the surface of articular cartilage was completely destructed and composed of disorganized fibrocartilage and fibrous tissues. In addition, the subchondral bone was detached (Figure 3E). After 12 weeks of collagenase intra-articular injection, the knee joint space was significantly wider in the synovial stem cells plus secreta group than in the Hyalgan and control groups. Furthermore, the secreta group demonstrated better results than the synovial stem cell group in terms of joint space width (Figure 3).
Radiological and histopathological findings showed similarity in which a significant enhancement of articular cartilage was seen in the synovial stem cell and secreta groups.
5. Discussion
The present study demonstrated considerable healing in the stem cell plus secreta-treated group when compared to the Hyalgan and non-treatment groups.
All joint tissues contain a certain amount of MSCs that can differentiate into joint structures such as the bone, cartilage, and even synovial membrane (7). In degenerative diseases such as osteoarthritis, MSCs in patients with end-stage status lose their functions both in vivo and in vitro. Due to the changes in the conditioned media supplements and alterations in MSCs growth factor receptors, the MSCs differentiation profile changes and adipogenic and chondrogenic differentiation capacity reduces, while the osteogenic differentiation capacity rises (18).
Mesenchymal stem cells have become a major tool in stem cell therapy in diseases that affect bone and joint function (19). The interest in using MSCs is proven due to the quick proliferation capacity, convenient isolation, potential differentiation to tissues of mesenchymal lineage like the bone, cartilage, and adipose, anti-inflammatory potential, immune-modulatory potential, biological active factor secretion, and tropic activity (20).
Tang et al. (21) evaluated the effects of subcutaneous A-MSCs injection in OA rats and showed that the cartilage surface was smooth along with the good distribution of chondrocytes, which confirms our findings. The continuous cartilage surface in the synovial stem cell group in the current study was in line with the findings by Chiang et al. (22) that reported the regularity of cartilage surface in joints treated with MSCs in OA and Singh et al. (23) findings that showed chondrocytes’ normal distribution and mild irregularity in the cartilage surface in BM-MSCs-treated rabbits with induced OA. These findings demonstrate the therapeutic effects of MSCs.
Our radiological results are similar to the findings by Toghraie et al. (24) that showed a reduction in both osteophyte formation and subchondral bone necrosis in OA rabbit treated with MSCs. Thus, MSCs could be promising cell sources for OA regeneration (24). In the early stage of osteoarthritis inflammation, secreted inflammatory cytokines can increase MSCs proliferation, but as inflammation exacerbates and inflammatory cytokines are flared up, native MSCs are suppressed dose-dependently (25). When inflammation progresses, inflammatory cytokines such as INFγ and TNFα induce alterations in the paracrine signaling of MSCs to increase IL-6, HGF, VEGF, and TGFβ, which are responsible for the immune-modulatory effect of MSCs, leading to the suppression of inflammation, biological activation of osteoblasts and chondrocytes, and joint healing (26, 27).
Although MSCs are the perfect sources for stem cell therapy in osteoarthritis, many recent studies have demonstrated that intra-articular-injected MSCs have a poor survival rate and differentiation potential, and native stem cells seem to be more efficient in joint regeneration than transplanted ones. Furthermore, cell signaling pathways alter after MSC injection. Based on the results mentioned above, many studies have suggested the principal benefit of stem cell therapy owing to paracrine activity (28-30).
Some studies have indicated that secreta can contrast with inducible NO synthase (iNOS), which is stimulated in chondrocytes by IL-1β and plays a major role in the articular destruction. Moreover, secreta can downregulate other inflammatory media like COX-2, PGE2, and MMPs by IL-1β inhibition (31). Herein, secreta treatment revealed normality in surface cartilage, chondrocyte distribution, and subchondral bone formation, which are supported by Li et al. (32) that reported the role of secreta in bone regeneration, osteogenesis promotion, and osteoblasts ossification potential enhancement.
Kuroda et al. (13) evaluated the effect of secreta of adipose-derived stem cells on OA in knee joints of rabbits and reported the milder progression in OA by the acceleration of cartilage formation process, viability of chondrocytes, and cartilage matrix production and protection, which confirm the results of the present study.
Our results are consistent with the findings obtained by Linero and Chaparro (25) that reported the facilitating of bone regeneration and cell migration, proliferation, and survival in rabbits with surgical jaw bone lesions due to the paracrine effect of human adipose tissue-derived mesenchymal stem cells. The above results indicate that the paracrine effect of synovial stem cells injected into intra-articular soft tissue contributes mainly to the prevention of cartilage deterioration advancement.
5.1. Conclusions
The intra-articular injection of synovial-derived stem cells with secreta may inhibit the cartilage degeneration process in rats with osteoarthritis by the secretion of anti-inflammatory agents and growth factors that increase the number and chondrogenesis activity of native mesenchymal stem cells.