1. Background
In recent years, few preclinical studies have signified the effect of Vitamin D3 (VitD3) and its active metabolite on cancer and vascular endothelial cells, which leads to cancer inhibition through apoptosis and growth cell arrest.
VitD3 enhances anti-cancer activity in some types of cytotoxic anticancer drugs in preclinical models. VitD3 and its active metabolites perform signaling innovation through several important pathways, but the essential pathway for VitD3 anti-tumor activity is unclear (1, 2).
The first report of the link between cancer risk and sun exposure was reported in 1941, but in recent years, many studies have reversed the relationship between sun exposure and prostate, breast, and colon cancers due to increased production of VitD3 by sunlight (3).
In many cancers, particularly renal cell carcinoma (RCC), low serum VitD3 levels lead to rapid tumor growth. Also, 1,25(OH)2VitD3 influenced the gap junctional intercellular communication (GJIC) during carcinogenesis. When cells were treated simultaneously with the VitD3 and tumor promoter, the GJIC functions remained at pretreatment levels.
These data suggest that lowering serum levels of VitD3 is a risk factor for cancer development and progression. VitD3 may also prevent RCC by maintaining the GJIC during carcinogenesis (4). Many studies have evaluated the effect of VitD3 in cancer prevention and control. The effect of VitD3 on cancer prevention and control as chemoprevention in high-risk patients has been reported in colon, breast, and prostate cancers (5-7).
Beyond the effect of VitD3 on calcium and bone metabolism, many in vitro studies have shown the efficacy of VitD3 and its metabolites on the induction of differentiation and apoptosis in epithelial tissues and also their effects on angiogenesis and proliferation.
High VitD3 intake inhibits carcinogenesis. Based on a trial and observational studies on VitD3 intake and serum levels of 25-(OH)VitD3, an inverse relationship was found between these measures and the risk of cancer, especially colon cancer and adenoma (8, 9).
The active form of VitD3, 1α,25-dihydroxy VitD3, acts as a physiological Vit D receptor ligand and regulates various physiological processes, such as calcium and bone metabolism, cell growth and differentiation, immunity, as well as cardiovascular function.
The secondary bile acid (lithocholic acid) is a natural Vit D receptor ligand that regulates calcium metabolism and immune and inflammatory responses. Vit D receptor ligand is promising in the treatment of cancer, autoimmune, and cardiovascular disease (10).
A limited number of studies have focused on the correlation between malignancy of hematopoietic system and VitD3 level.
The results of a study on acute myeloid leukemia patients showed that serum 25 (OH)VitD3 deficiency was very common in this group. In addition, higher levels of VitD3 in these patients were associated with better outcomes (11).
2. Method
2.1. Study Design
The present prospective cohort study was done to determine the levels of 25(OH)VitD3 in patients diagnosed with chronic lymphocytic leukemia (CLL). This cohort study was conducted during a one-year period from March 2016 to March 2017 at the medical oncology and hematology department of Namazi Hospital affiliated to the Shiraz University of Medical Sciences.
The study protocol was approved by the Research Ethics Committee of Shiraz University of Medical Sciences and followed the principles stated in the Helsinki Declaration. Informed consent was obtained from all participants in the study.
2.2. Patients
A simple sampling method was used, and all new and old diagnosed patients with CLL above 18 years old were referred to the medical oncology and hematology clinic, and the hospital entered the study. Finally, 86 patients were included in the study.
In a cross-sectional study and according to the patients' history, 86 CLL patients were evaluated for the relationship between VitD3 serum levels and Rai stages, age, gender, pathological factors, CD38, and ZAP-70. Further, to compare the VitD3 levels of CLL patients with the normal population, a control group was added to the study. The Rai stages were according to the laboratory and clinical findings; ZAP-70 and CD38 were measured using flow-cytometry methods. VitD3 level was assayed using the immunoassay technique. Mann-Whitney U test, Kruskal-Wallis test, and Pearson’s correlation were estimated with 95% confidence interval.
2.3. Serum 25(OH)D Levels Measurement
Serum level of 25(OH)VitD3 was assessed by venous blood samples that were taken from each patient in the first referral to the hematology and medical oncology clinic. Blood samples were centrifuged, and serum extraction was done, and serum 25(OH)VitD3 concentrations were measured by enzyme-linked immunosorbent assay (ELISA) method.
2.4. Data Collection
Baseline patients' demographic data, including sex, age, major complain, and peripheral blood flow cytometry for confirmation of CLL, was taken. Laboratory parameters, including complete blood count (CBC), imaging studies for evaluation of spleen size, and other parameters, such as ZAP70 and CD38, were evaluated. All patients were staged according to the Rai staging system into five groups. Secondary complications, including bacterial and viral infections, hemolysis, and second primary cancer, were also recorded.
2.5. Statistical Analysis
Statistical analysis was performed using SPSS software version 22. Continuous variables were presented as mean ± standard deviation (SD) and median (IQR) with non-parametric tests, such as the Mann-Whitney U test according to data distribution specifications. Classified variables were reported in frequencies (percentage). The differences between the classification variables were analyzed with the Chi-square and Fisher’s exact tests. Correlations were searched using Pearson’s and Spearman’s coefficients for variables with normal and abnormal distributions, respectively. For all analyses, a P < 0.05 was considered significant.
2.6. Ethics Statements
In this study, all steps related to patients’ participation were performed in accordance with the ethical standards of the Research Committee and the 1964 Helsinki Declaration and its subsequent amendments or comparable ethical standards. This study was approved by the ethics committee of Shiraz University of Medical Sciences, Shiraz, Iran (code: IR.SUMS.MED.REC. 1395.s191).
3. Results
A total of 86 patients (58 males and 28 females) participated in our study. The age range of patients included in this study was 32-86 years old. The median age of men and women was 66.63 ± 10.96 and 65.81 ± 9.48 years, respectively. The Pearson correlation of 0.451 showed no correlation between serum 25(OH)VitD3 level and patients’ age (P = 0.088).
The mean serum level of 25(OH) VitD3 in patients with CLL was 28.666 ± 17.528 ng/mL. The mean serum level of 25(OH)VitD3 in men with CLL was 27.884±13.537 ng/mL and in women was 30.281±24.341 ng/mL. The highest and lowest level of serum 25(OH)Vit D3 was 122 and 7.1 ng/mL, respectively. According to the Mann-Whitney U test, the level of serum 25 (OH)VitD3 between men and women with CLL was not statistically significant. Considering the normal value of serum 25(OH)VitD3 (30-150 ng/mL) in the normal population, the results showed that 69.8% of patients with CLL had 25(OH)Vit D3 serum levels below 30ng/mL, and only 30.2% of patients had a normal value of serum 25 (OH)Vit D3.
Also, about 50% of patients had extremely high serum 25 (OH)Vit D3 levels of around 28ng/mL, and 90% had serum 25(OH)VitD3 levels of about 48 ng/mL.
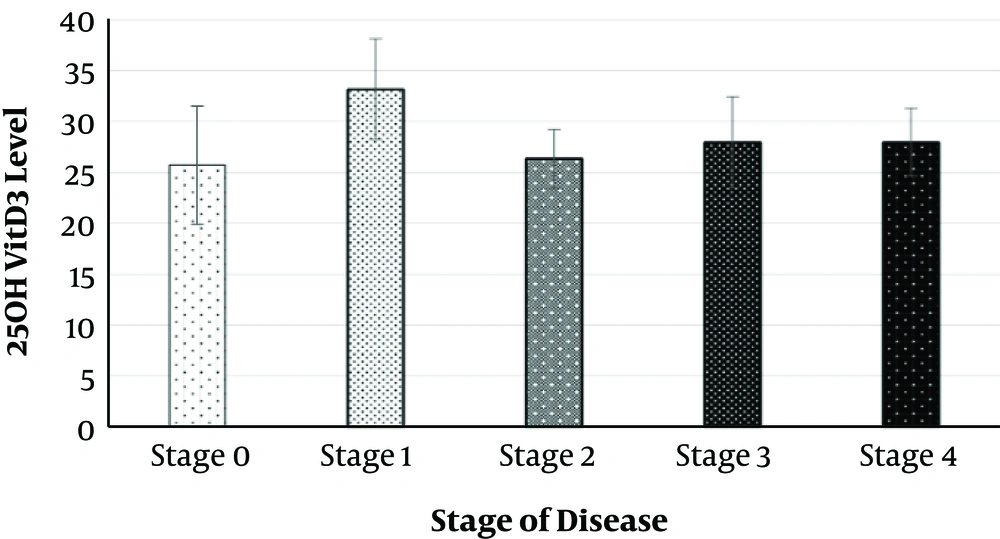
The abundance stage of the disease (stage 0) in CLL patients is shown in Figure 1, and the majority of patients were in stage 1 and 2. Stages 2 and 4 were more common in men, which in comparison with women, there was the same distribution regarding stages 0 to 3. The results of the Chi-square test showed a significant difference between men and women in terms of the disease stage (P < 0.001).
The highest level of serum 25 (OH)VitD3 level was in stage 1 and the lowest was in stage 2. However, according to the one-way ANOVA results, there was no significant relationship between serum 25 (OH)VitD3 level and stage of disease (P = 0.68).
Regarding prognostic factors, there was no significant difference in the CD38 and ZAP70 expression in patients with and without VitD3 deficiency. Concerning the secondary complications of CLL, including bacterial and viral infections, hemolysis, and second primary cancer, 16 patients (18.82%) had a secondary complication that was not significant between the two groups (P = 0.125).
According to primary white blood cell (WBC) count and high doubling time, all patients were divided into three groups; the first group with WBC < 10,000, the second group with WBC of 10,000 - 30,000, and the third group with WBC > 30,000. According to the Kruskal-Wallis test, there was no significant difference between serum VitD3 level and WBC count, and high doubling time (P = 0.785).
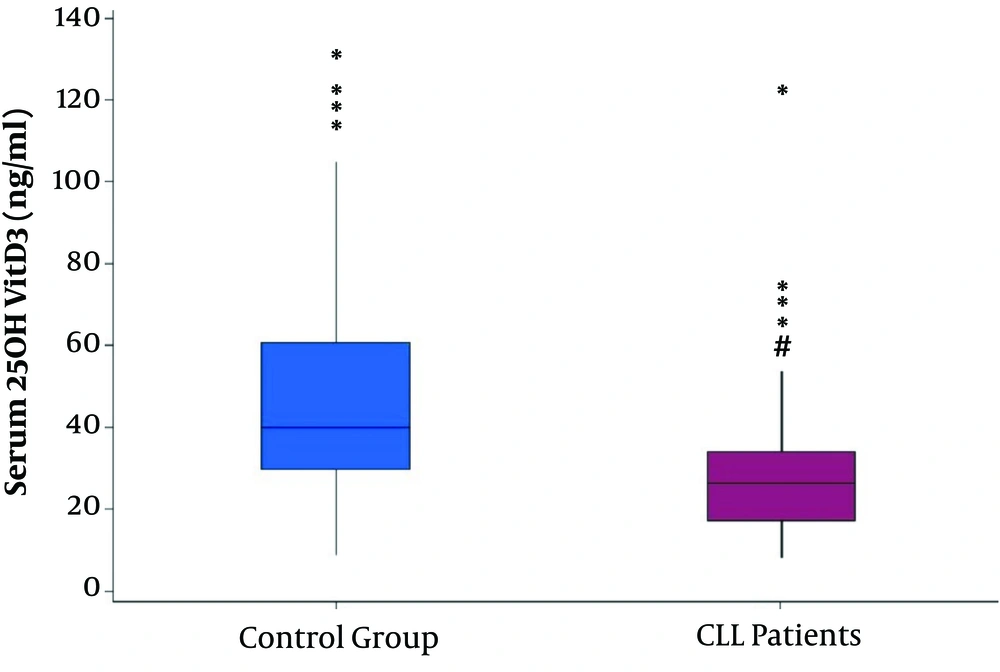
In this study, serum 25(OH)VitD3 in CLL patients was compared with the control group of the normal population. In the control group, 111 cases, including 62 men and 49 women were selected randomly from the normal population. The age range and mean age of the control group were completely similar to the CLL group (22 - 93) 61.17 ± 10.73. The mean 25(OH)VitD3 level in the control group was 47.77 ± 25.69 ng/ml, which was significantly higher than the CLL group (28.66 ± 17.52 ng/mL; P = 0.001) (Figure 2).
Considering the normal range for serum 25(OH)VitD3 (30 - 150 ng/mL), 27% of the control group had 25(OH)VitD3 levels below 30ng/mL that was comparable with 69.8% in the CLL group.
4. Discussion
Deficiency of VitD3 has been reported previously to increase the risk of many solid tumors, including breast, colon, prostate, as well as hematologic malignancies (5-7, 11). The optimal dose of VitD3 that has a significant anticancer effect is 400 IU, which has been mentioned in previous articles. Higher doses may be harmful or promote cancer (12). The serum concentration of 25(OH)VitD3 not only influence the tumor production but also can affect tumor growth and progression (12, 13).
In addition, VitD3 prevents the proliferation of T helper cells and inhibits CD4 + T cell cytokines, which play an important role in preventing and controlling inflammatory and immune responses (14).
The major question here is the safety of VitD3 supplements in CLL and whether it should be recommended for the patients. Kubeczko et al. in 2016 reported that in CLL patients with VitD3 deficiency due to inferior prognosis, VitD3 prescription is safe and can be effectively recommended (15).
In this study, we observed no correlation between sex, age, stage, prognostic factors, and secondary complications, and VitD3 serum levels. However, VitD3 serum level in CLL patients was lower than in healthy normal population. This means that VitD3 deficiency is not effective in disease progression and outcome, but it may be an etiologic factor that may or may not be the cause of the disease. The prevalence of VitD3 deficiency in the Iranian healthy population and other people is an important issue and the comparison between patients and the healthy population seems necessary. In a study by Khosravi-Boroujeni, the prevalence of VitD3 deficiency in the Iranian healthy population was reported from 24.4 - 30.5%, and the mean serum VitD3 levels were 52.12 - 62.28 nmol/L. Also, the risk of VitD3 deficiency decreased from 2001 to 2013 (3).
The protective effect of VitD3 against cancer has been mentioned in various studies; however, no accepted clinical evidence is available. Although molecular and cell culture models support such effect of VitD3, clinical studies showing such a protective effect against at least one type of cancer are rare (16).
It is very hard to consider VitD3 deficiency as a major risk factor for CLL patients but according to previous studies, low serum VitD3 may be instrumental in developing cancer. Furthermore, epidemiological data showed that measurement of serum 25(OH)VitD3 level is used as a risk assessment factor in early diagnosis, prognosis, and treatment of many cancers and is promising in the future of cancer treatment (15).
The effect of VitD3 and its association with prognostic factors in non-Hodgkin's and CLL lymphoma malignancies have been investigated, and finally, it was found that VitD3 levels are significantly low in patients with CLL and diffuse large B-cell lymphoma (DLBCL) and it may be associated with advanced stages (17).
In this study, we found that VitD3 deficiency is more common in CLL patients in comparison with the normal population. The most prevalent stages in this study were stages 1 and 2 in about 52.32% of all patients with CLL. Overall, CLL was more prevalent and more aggressive in men than women because advanced and higher stages were more common in men.
However, in the current research, there was no significant relationship between VitD3 serum levels and investigated variables, except splenomegaly in CLL patients; this finding is consistent with other studies. However, including a control group in this study resulted in better findings. Therefore, it is suggested that clinical trials be designed for clarifying the real effect of VitD3 in clinical settings.