1. Context
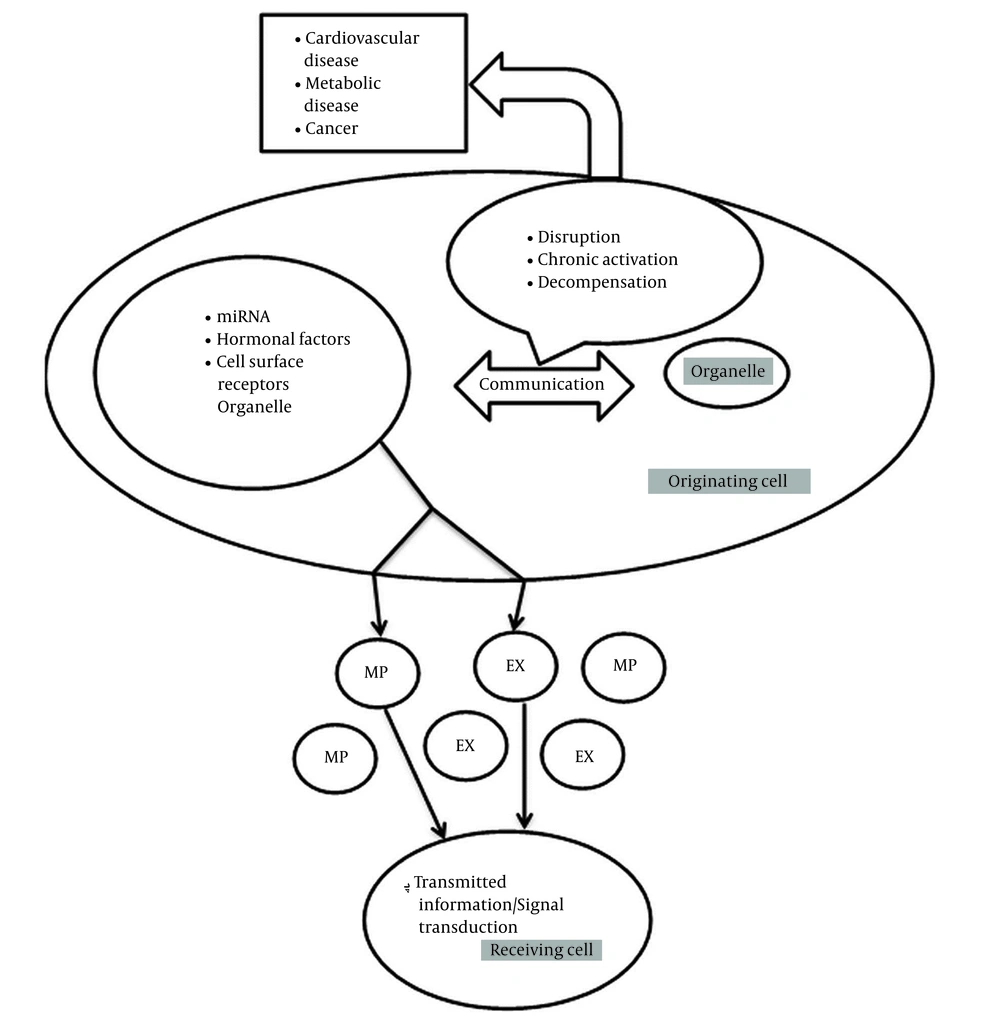
Cell-to-cell communication mediated by extracellular vesicles is an emerging biological concept. Extracellular vesicles are shed by almost all cells and are subclassified as exosomes and microparticles (MPs) according to their size. For example, for glomerular cells to function as an integrated filtration unit, cell-to-cell communication or crosstalk is required (1). Subcellular or intracellular organelles in eukaryotic cells connect with other organelles for cell function. Cellular organelles contain nucleus, mitochondria (mt), endoplasmic reticulum (ER), peroxisomes, and lysosomes with properties of organelle biogenesis. Subcellular organelle biogenesis is indicative of metabolic activities in cell, and any derangement in physiologic function results in pathologic state (2). In addition to organelle-to-organelle communication within the cell, autocrine, paracrine, and even endocrine mechanisms can be transmitted via extracellular vesicles such as microparticles and exosomes. These organelles convey mitochondrial ribonucleic acid (miRNAs), hormonal factors, and cell surface receptors from original cells to receiving cells, and their function is transmitting information or signal transduction in this network (Figure 1) (3, 4). Therefore, dysfunction of mitochondrial crosstalk with other organelles is associated with an increasingly large proportion of human inherited disorders and is related to diseases, such as neurodegenerative disorders, cardiomyopathies, metabolic syndrome, cancer, and obesity (5).
2. Objectives
The aim of this research is to identify the effect of interorganelle communication (crosstalk) on physiopathological states of cells in health and disease.
Cells are dependent on a compartmentalized system for biochemical processes and signaling response to survive and develop. Mitochondria contain double membranes with deoxyribonucleic acid (DNA) in their matrix. They produce cell energy through oxidative phosphorylation and involve in the majority of metabolic processes and enzymatic pathways. Herein, every eukaryotic cell consists of essential signaling process that transmits material between organelles. Furthermore, interorganelle crosstalk is executed as specialized membrane contact sites (MCSs) through protein-protein and lipid-protein transports. It is worth noting that exploring these interactions can lead to the discovery and treating new diseases.
Mitochondrion is one of the cellular organelles that performs functions of metabolic process, adenosine triphosphate (ATP) production, cell death decision, and immune signaling. Mitochondrial crosstalk with other organelles are essential for cell biology and are known to regulate crucial cellular processes. Dysregulation of exchangeable metabolites in mitochondrial crosstalk with other organelles seems to have a role in the etiology of diseases.This study aimed to evaluate how crosstalk between mitochondria and other organelles can cause clinical effects (disease).
3. Methods
3.1. Inclusion Criteria
3.1.1. Type of Articles
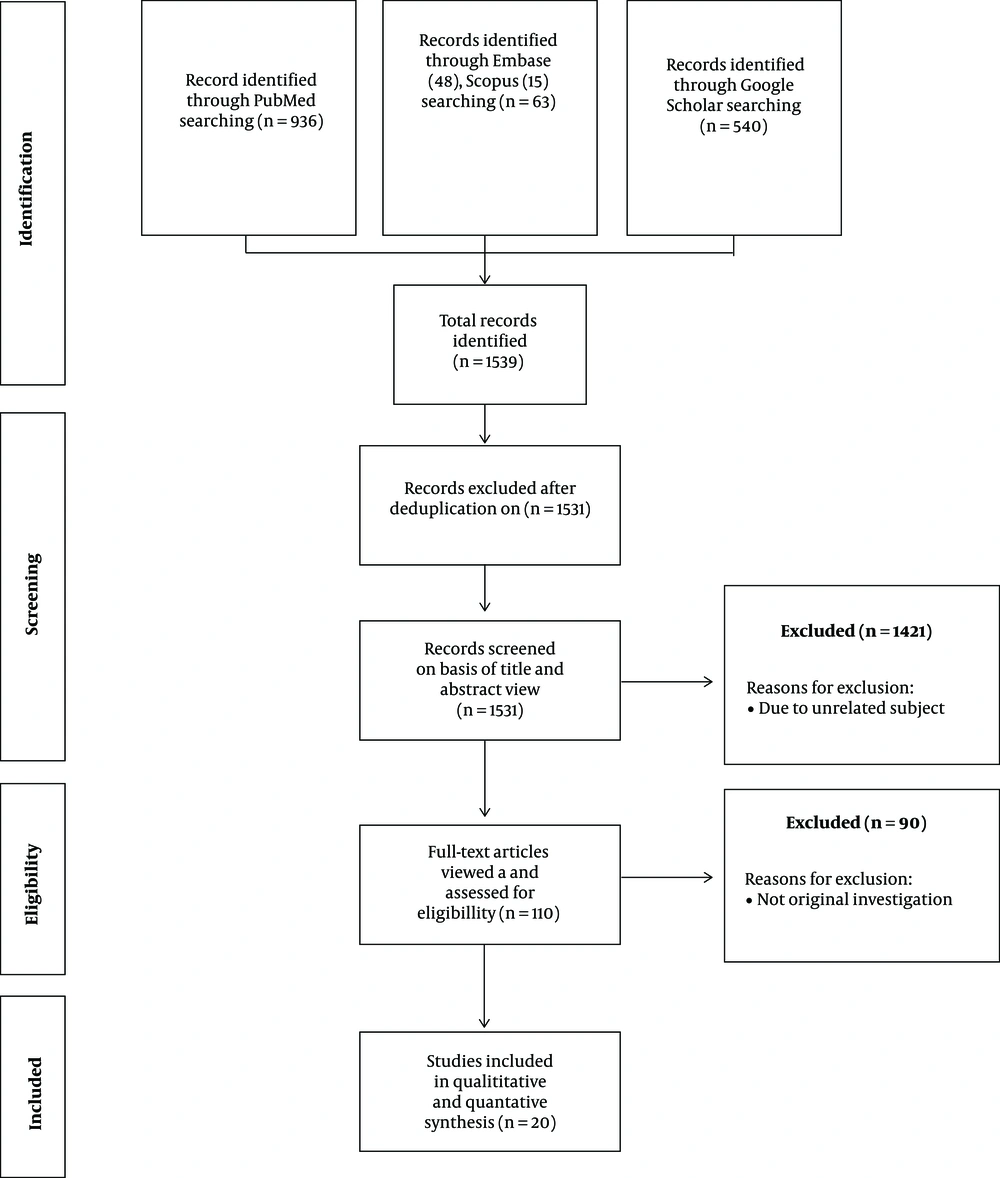
Out of a total of 1539 articles identified in this study, eight articles were duplicated. Also, 1421 articles were removed due to unrelated subject. Then, 110 full-text articles were identified as eligible for review. Next, 90 articles were excluded due to originality problems and irrelevance to interorganelle communication. Finally, 20 original and research articles were obtained via electronic search in PubMed Central (PMC), Embase, Scopus, and Google Scholar databases. The included articles examined the effect of interorganelle communication on disease generation.
3.1.2. Type of Participants
All the included studies were original or research articles investigating interorganelle crosstalk and communication.
3.1.3. Type of Outcome Measures
3.1.3.1. Primary Outcomes
Primary end-points of interorganelle crosstalk contained clinical end-points and pharmacologic inhibition of molecules or signaling pathways.
3.2. Databases
The articles were found based on advanced searching in PubMed Central, Embase, Scopus, and Google Scholar databases up to April 2020.
3.3. Search Strategy
This search strategy was categorized as analytical, clinical studies (experimental) and was designed as randomized clinical trials. A total of 1,539 articles were identified based on title or abstract screening; eight papers were removed due to deduplication. The retrieved studies investigated the following issues: interorganelle communication (N = 1,463), interorganelle crosstalk (N = 34), mitochondria-endoplasmic reticulum crosstalk (N = 20), mitochondria-nucleus crosstalk (N = 8), mitochondria-peroxisome crosstalk (N = 6), and mitochondria-lysosome crosstalk (N = 8). The following search terms and their combination were used: Boolean operator AND: mitochondria and endoplasmic reticulum crosstalk, mitochondria and nucleus crosstalk, mitochondria and peroxisome crosstalk, mitochondria and lysosome crosstalk.
3.4. Searching Other Resources
The author reviewed the references of all included articles and handsearched them to identify additional relevant articles.
3.5. Study Selection
The search strategy was used to obtain titles and abstracts of studies that might be relevant to the review. As a result, 936, 48, 15, and 540 titles and abstracts were retrieved from PubMed Central, Embase, Scopus, and Google Scholar, respectively. Total records of 1539 articles were screened, and 1531 articles were identified after deduplication. Then, 1421 articles were excluded due to non-related subject, review articles, and others, and eventually, 110 full-text articles were considered for eligibility. Next, 90 articles were removed due to originality problems and irrelevance to interorganelle communication. Finally, 20 articles were included in the meta-analysis.
3.6. Data Collection and Analysis
3.6.1. Data Extraction
Data extraction was carried out, and non-English articles were translated before assessment. Where more than one publication of one study existed, reports were grouped together, and the publication with the most complete data was included. Data gathered from each article included the following items: title, first author, journal, year of publication, location, type of clinical study, study design, period of intervention, characteristics of the intervention, and control.
The extracted data yielded functions of crosstalk between mitochondria and other organelles that were obtained in research laboratories experimentally. These functions include Ca2+ homeostasis, apoptosis and autophagy, reactive oxygen species (ROS), adenosine triphosphate (ATP), redox state, lipid metabolism, and respiratory chain (RC) dysfunction. These variables were measured in this research.
3.6.2. Quality Assessment and Risk of Bias
The Cochrane collaboration’s tool was used for assessing risk of bias (CROB). All included articles were assessed in seven areas (random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, and other biases) as proposed by Cochrane. Some assessment tools were introduced as meta-bias (publication bias and funding source). For this analysis, the CROB categories were dichotomized by recording low risk as 1 and high or unclear risk as 0.
3.6.3. Summary Measures
Effect size of interorganelle crosstalk on various variables was defined as percentage (event rate). Percentage of values was assessed as row, column, and table percentages. The relative risk of disease was defined as relative ratio of disease in two groups of exposed with and without disease with risk factor (interorganelle crosstalk). Odds ratio was defined as the ratio of odds in two groups of exposed with and without disease with risk factor. Effect measures of disease were assessed with comparison of risk or odds of disease between two groups with and without disease in exposure to risk factor.
3.6.4. Statistical Analysis
All categorical variables were expressed as frequency and percentage (ratio). Percentage difference was assessed for comparison between two groups. Relative risk, odds ratio, and 95% confidence interval were used for binary outcomes. Effect size of two groups were done by assessing odds ratio, and estimated effect size (prevalence or event rate) of variables were assessed as the proportion of one variable (e.g., variable A) in a population (e.g., population B). Comparison between categorical groups was done using the chi-square test, and comparison between binary outcomes was done using Fisher’s exact test (for number of variables less than 5). Q (chi-square) and I2) tests were used for assessing CROB and heterogeneity. Significance was assessed with a p-value of < 0.05 (Appendix 1).
4. Results
4.1. Description of Articles
The author identified 1539 records after searching via electronic databases. After removing duplicated articles, 1531 articles were screened based on titles and abstracts. Then 1421 articles were discarded due to unrelated subjects, and 110 articles become eligible for review. Thereafter, 90
articles were discarded due to not original investigations. Of these, 20 published articles were included and enrolled in this study (Figure. 2).
4.2. Study Design
In this prospective research, the existing data were typed as analytic clinical studies (experimental) and planned with randomized clinical trials. Finally, experimental studies were collected.
4.3. Setting
All studies were performed in the research laboratories of university or medical institutions.
4.4. Exclusion Criteria
Any review article or letter to the editor in case of interorganelle communication was excluded from the research.
4.5. Risk of Bias in the Included Studies
All included articles were assessed using the Cochrane collaboration's tool, and a ranking of high, low, or unclear risk was given to each paper in seven areas of CROB. The studies containing insufficient data, outcomes, or clinical entity could not be assessed accurately due to the risk of bias. Methodological details of the studies were not completely reported in the included studies. One article did not report the methodological details of the study. In three studies, statistical analyses had not been reported separately. In none of the studies, outcomes had been described accurately. Moreover, reporting of data in two studies was incomplete. In other words, in this research, 75% of studies had a low risk of selection, and detection biases, and 25% of studies had a high risk of selection bias. Performance bias was low in 95% and high in 5% of studies. Reporting and attrition biases were low in 80%, and detection, attrition, and reporting biases were high in 10% of studies. Other biases were low in 10%, high in 75%, and unclear in 15% of studies. Funding source (meta-bias) was characterized in 90% of studies, and it had not been characterized in 10% of studies. Publication bias were unclear in one hundred percentages (100%) of included articles. The Q test (using chi-square test) for these studies was 82.6 with freedom degree (df) of 7 and significance level of 0.05 (P = 3.55). Furthermore, I2 heterogeneity (Q > df) was quantified 91.5% for these studies. This value means presence of considerable heterogeneity between studies in research (Appendix 2).
4.6. Results of Case Studies
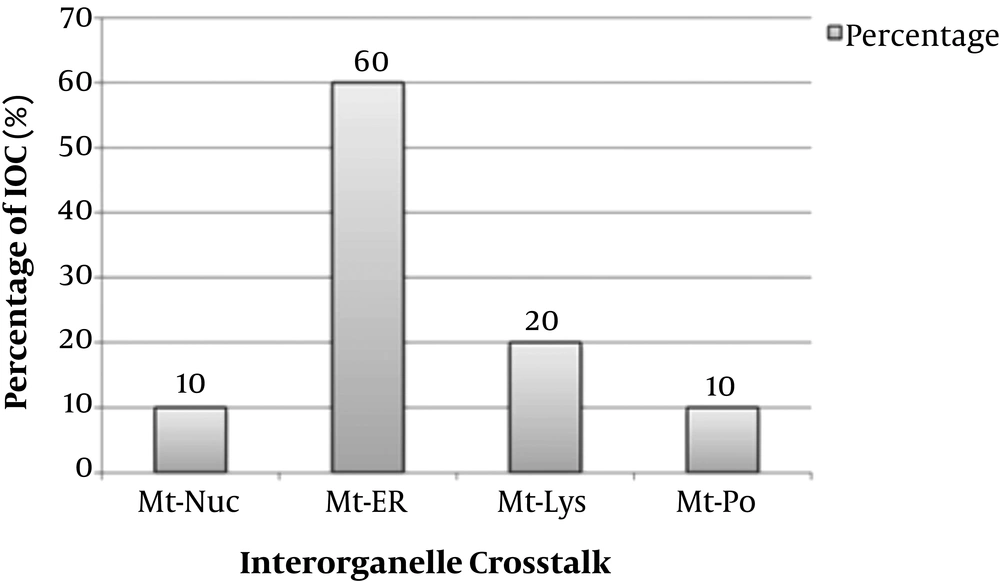
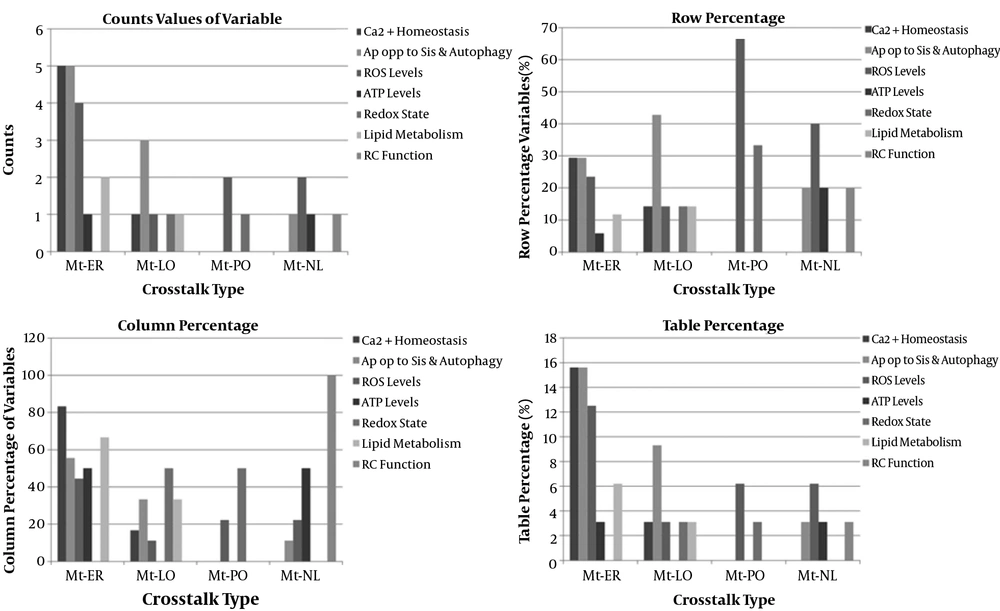
The strategy recovered 139 unique records after deduplication. Of these, 110 articles were eligible for this meta-analysis, and twenty studies included in this research. The 20 studies examined 20 mitochondrial communication with other organelles (6-25). The details of included studies were depicted in Appendix 3 - 23. Twelve studies were related to mitochondria-endoplasmic reticulum crosstalk (12/20, 60%), four studies were related to mitochondria-lysosome (4/20, 20%), two studies were related to mitochondria-nucleus crosstalk (2/20, 10%), and two studies were related to mitochondria-peroxisome crosstalk (2/20, 10%) (Figure 3). Studies were searched since inception to April 2020 years, and twelve studies were from Europe (60%), six studies from America (30%), and two studies from Asia (10%). The included studies comprised eleven animal experimental studies (11/20 or 55%), three human experimental studies (3/20 or 15%), one human and animal cohort study (1/20 or 5%), and five animal and human experimental studies (5/20 or 25%). Interorganelle communication and crosstalk enrolled in calcium homeostasis, apoptosis and autophagy, ROS, ATP production, redox state, RC function, and lipid metabolism. Apoptosis and autophagy enrolled in five of nine (5/9, 55.5%) studies of mitochondria-ER crosstalk, three of nine (3/9, 33.3%) mitochondria-lysosomal communication studies, and one of nine (1/9, 11.1%) mitochondria-nuclear communication studies. Apoptosis was not observed in studies of mitochondria-peroxisome crosstalk (0/9). Calcium homeostasis had been investigated in five of six (5/6, 83.8%) mitochondria-ER communication studies and one of six (1/6, 16.6%) mitochondria-lysosomal communication studies. Increased ROS levels found in two of nine (2/9, 22.2%) mitochondria-nucleus and mitochondria-peroxisome, four of nine (4/9, 44.4%) mitochondria-ER studies, and one of nine (1/9, 11.1%) mitochondria-lysosomal communication studies. Declined ATP levels were found in one of two (1/2, 50%) mitochondria-nucleus communication and mitochondria-ER crosstalk studies. Redox state was seen in one of two (1/2, 50%) mitochondria-PO and mitochondria-lysosomal communication studies. Lipid metabolism was found in two of three (2/3, 66.6%) mitochondria-ER and one of three (1/3, 33.3%) mitochondria-lysosomal communication studies. RC dysfunction was found in one of one (1/1, 100%) mitochondria-nuclear communication studies (Appendix 24) (Figure 4). Estimated effect size (proportion) of mitochondria-ER, mitochondria-nucleus, mitochondria-peroxisome, and mitochondria-lysosome crosstalks on increased ROS levels were assessed 33.3%, 100%, 100%, and 25%, respectively. Proportion difference of increased ROS levels in mitochondria to ER, mitochondria to nucleus, mitochondria to peroxisome, and mitochondria to lysosome crosstalks were assessed 29.2%, 36.4%, 36.4%, and 25%, respectively. These values based on RR and ORs included 0.53, 0.02, 0.02, 0.5, and 0.32, 0, 0, and 0.33, respectively. Comparison of Odds ratio of increased ROS levels with control group was assessed with p-value of 0.2 and 0.38 using chi-square test, respectively. Effect size (proportion) of mitochondria-ER, mitochondria-nucleus, mitochondria-lysosome, and mitochondria-peroxisome crosstalks on apoptosis were assessed 41.6%, 50%, 0%, and 75%, respectively. These values as relative risk were assessed 0.83, 1.13, 0.0, 2 and as odds ratio were assessed 0.71, 1.25, 0, 5, respectively. Comparison of odds ratio of apoptosis with control group for Mt-ER, Mt-Nuc, and Mt-Lys were assessed with p-value of 0.71, 0.88, and 0.20 using chi-square test, respectively (Appendix 25, Appendix 26). The relative risk of mitochondria to lysosome crosstalks on apoptosis showed positive association, and odds ratio of mitochondria to lysosome crosstalks on apoptosis was revealed as large effect.
Percentage of Different Types of Mitochondrial Crosstalk with Other Organelles in Medical Science. IOC, interorganelle crosstalk; Mt-ER, mitochondria-endoplasmic reticulum crosstalk; Mt-Lys, mitochondria-lysosome crosstalk; Mt-Nuc, mitochondria-nucleus crosstalk; Mt-PO, mitochondria-peroxisome crosstalk.
Percentage of different functions of mitochondrial crosstalk with other organelles in medical science. ATP, adenosine triphosphate; ca2+ homeostasis, calcium; er, endoplasmic reticulum; lo, lysosome; nl, nucleus; mt, mitochondria; po, peroxisome; rc function, respiratory chain function; ros, reactive oxidative species.
4.7. Outcomes
4.7.1. Primary End-Points
Outcomes of interorganelle crosstalk in the present study included clinical end-points (glomerular disease, cardiovascular disease, metabolic syndrome, metabolic disease or disorders, cancer, neurologic disease, aging, and age-related disorders) and pharmacologic inhibition of molecules or signaling pathways.
4.7.2. Clinical Entity
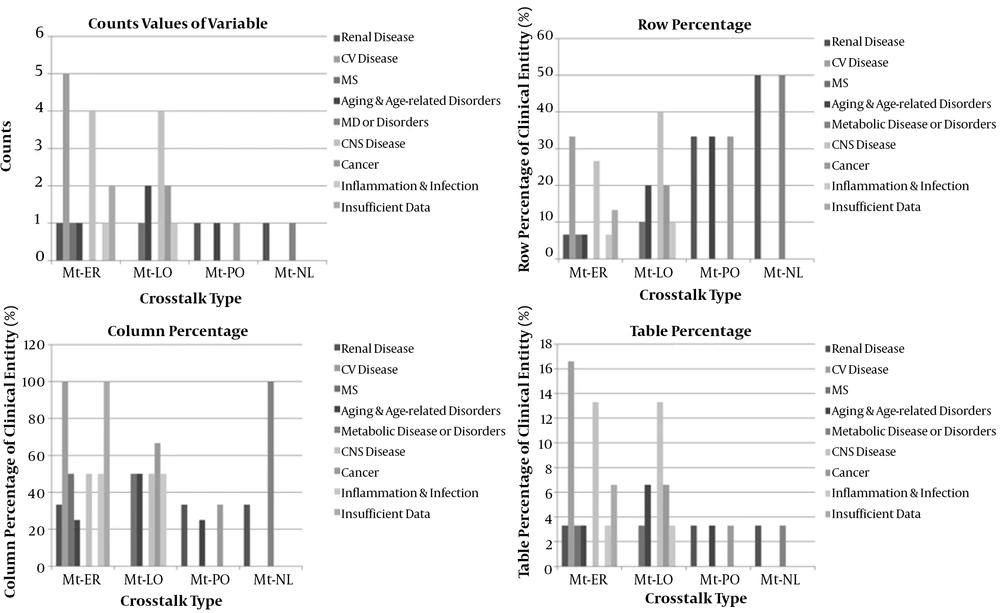
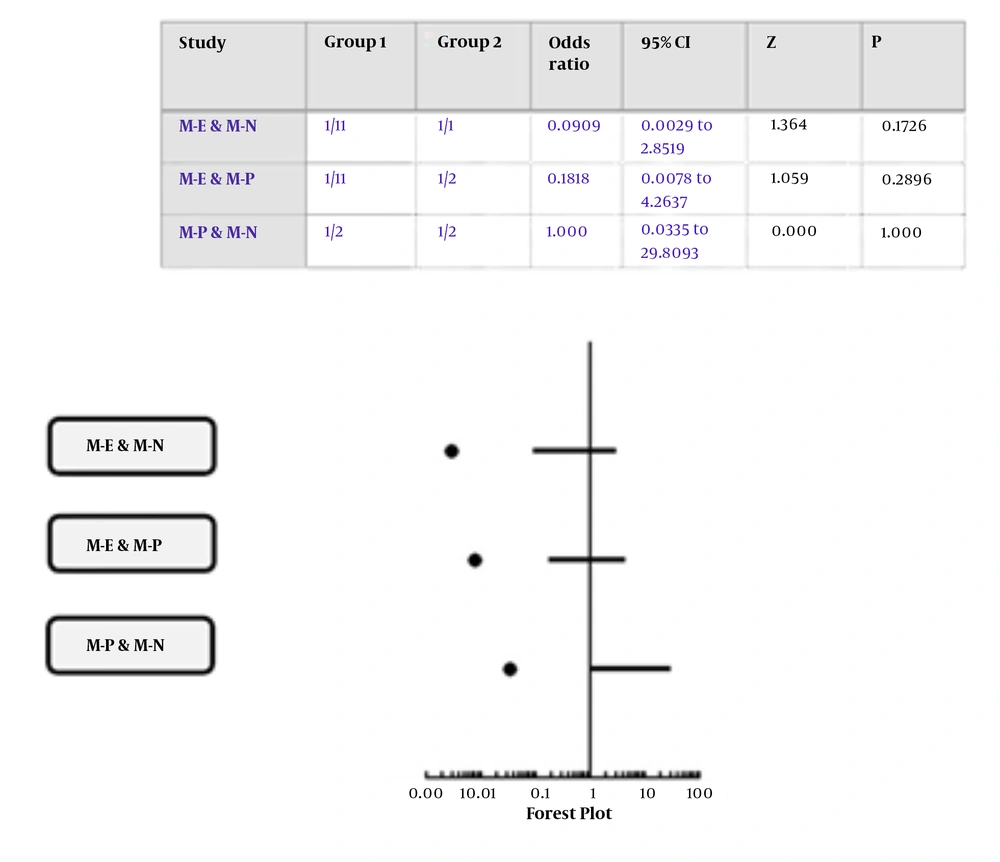
In the present study, four of thirty diseases (13.3%) correlated to central nervous system disease (CNS), five of thirty diseases (16.6%) attributed to cardiovascular disease, one of thirty diseases (3.3%) related to aging (and age-related disorders), renal disease, metabolic syndrome, inflammation (infection), and metabolic disease or disorders. Moreover, there was insufficient data in two of thirty diseases (6.6%) in interorganelle crosstalk in the present research (Appendix 27) (Figure 5). Interorganelle communication between mitochondria and ER or nucleus did not have an effect on renal disease as outcome of this relationship [RR of 0.17, OR of 0.09; 95% CI: 0.0-2.85; P = 0.17]. Moreover, comparison of this outcome between mitochondria with ER or nucleus in accordance with chi-square test was 2.43 with p-value of 0.11, which was not significant. Relative risk for renal disorders in crosstalk between mitochondria and ER or PO was 0.25, OR of 0.18, with 95% CI of 0.01-4.26, and P = 0.25. Comparison between interorganelle crosstalk between mitochondria and ER or PO based on chi-square test was 1.29 with p-value of 0.25 (not significant). Relative risk for interorganelle crosstalk between mitochondria and nucleus or PO was 1, OR of 1, 95% CI of 0.03-29.8, and p-value of 1 (Figure 6). Comparison of interorganelle crosstalk between mitochondria and or ER did not show an effect on metabolic syndrome (RR=0.42, OR=0.36; 95% CI: 0.02-7.29; P = 0.49). Comparison of interorganelle crosstalk between mitochondria and ER or lysosome in accordance with chi-square test was 0.46 with P-value of 0.49 (not significant). The RR and OR for mitochondrial communication between peroxisome or lysosome for cancer were 1, 95% CI of 0.05 - 18.9, and p value of 1. Comparison of interorganelle crosstalk between mitochondria and peroxisome or lysosome in accordance with chi-square test was zero with p-value of 1 (not significant). Interorganelle crosstalk between mitochondria and ER or lysosome did not have an effect on CNS disease (RR = 0.67, OR = 0.5; 95% CI: 0.08-3.13; P = 0.45). Comparison of interorganelle crosstalk between mitochondria and ER or lysosome in accordance with chi-square test was 0.55 with p-value of 0.45 (not significant). Interorganelle crosstalk between mitochondria and peroxisome or lysosome had risk of 1.5 and odds of 2 on aging and age-related disorders (RR=1.5, OR=2; 95% CI: 0.07-51.5; P = 0.67), but it was not significant. Comparison of interorganelle crosstalk between mitochondria and PO or lysosome in accordance with chi-square test was 0.17 with P-value of 0.67 (not significant).
4.7.3. Pharmacological Inhibition of Involved Pathways or Molecules in Interorganelle Crosstalk
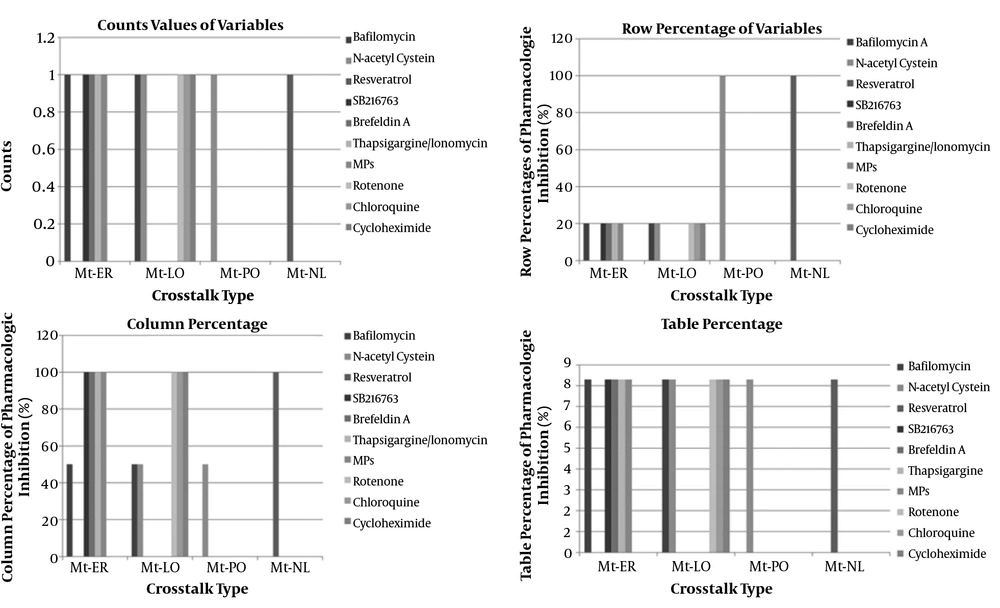
Our study showed that one of twelve studies (8.3%) was related to pharmacologic inhibition of two organelle crosstalk by N-acetyl cysteine, that is indicative of ROS-mediated pathways. Results of one study revealed that catalase deficiency enhances mitochondrial ROS and fibronectin expression in response to free fatty acids. The mentioned deficiency was effectively restored by catalase overexpression or N-acetylcysteine. This finding previously described and mentioned that S100A8/A9 causes cell death promotion through mitochondrial crosstalk with lysosomes by ROS and ∆TM-BNIP3 overexpression or N-acetylcystein co-treatment with S100A8/A9 decrease lysosomal activation in cells. Moreover, pharmacologic inhibition of one organelle crosstalk by bafilomycin one of twelve studies (1/12, 8.3%) indicated ROS pathways involvement by mitochondria-ER crosstalk and apoptosis pathways involvement by mitochondria-lysosome crosstalk in two different entities of infection and cancer, respectively. Collectively, it might be said that ROS pathways roughly involve in diseases such as infection, renal disease (DN), cancer, metabolic syndrome, CNS disease, aging, and age-related disorders (Appendix 28) (Figure 7).
5. Discussion
Mitochondria are energy powerhouse of cells and are important sites for cellular signaling in crosstalk with other organelles. They connect with the ER, peroxisomes, and nucleus via signal transduction, vesicle transport, and membrane contact sites to regulate energy metabolism, biosynthesis, immune response, and cell turnover. Thus, expressing mitochondrial communication with the other organelles of the cell is often a matter of survival or death as such mitochondria are constantly interconnecting with other organelles through signaling pathways and physical contact sites. Mitochondria and nucleus in cell communicate with each other via anterograde and retrograde signaling in order to adapt and coordinate their activities to environmental signals. New investigations suggest that non-coding ribonucleic acids (ncRNAs) might also play a role in this process (26). In context of mitochondrial crosstalk with nucleus, mitochondria biogenesis should be noticed because its modulation is carried out by the nucleus and the mitochondria genome. Regulatory mechanisms between two organelles are needed to adjust mitochondrial functions. Interorganelle crosstalk between mitochondria and nucleus in pathologic state leads to DNA damage in organelles, calcium overload, abnormal activation of growth factors, and metabolic disorders in carcinoma (27). Furthermore, interorganelle crosstalk is carried out via myriad ways such as MCSs that transfer metabolites, lipids, and proteins to other organelles and involve in organelle biogenesis and division (28). MCSs between mitochondria and endoplasmic reticulum have impact on autophagy initiation, mitochondrial division, and involve in Ca2+ homeostasis. Several mechanisms connect the unfolded protein response (UPR) due to ER stress and mitochondrial function, such as the regulation of mitochondrial fusion and fission. In a study by Inoue et al., silencing of protein-kinase-RNA-like-ER kinase (PERK), a kinase that mediates certain aspects of ER stress signaling, caused an increase in the apoptosis of Mfn-/- cells due to ER stress. Advantages of PERK silencing in these cells were low ROS production, normalized mitochondrial calcium, and improved mitochondrial morphology. Furthermore, loss-of-function of X-box binding protein-1), another transcription factor mediating the UPR, ameliorated autophagic activity of these cells upon ER stress (29). In continuation of this communication, the peroxisomes receive lipid and protein molecules from ER and contribute DRP1 (dynamin-related protein 1, a key fission regulator), Fis 1, and other proteins to mitochondria in division process. In other words, mitochondria express Fis 1, mitochondrial fission factor (MFF) as DRP1 receptors, whilst peroxisomes have specific DRP1 receptors, namely Peroxin (Pex 11). This receptor in peroxisomes seems to modulate interorganelle signals and serves as a tether molecule. The mitochondria-peroxisome contact sites seem to enhance the efficiency of cholesterol transport from peroxisomes to mitochondria during steroid hormone synthesis. Another metabolic process taking place in both mitochondria and peroxisome is the β-oxidation of fatty acids. Furthermore, other protein in interorganelle crosstalk between mitochondria and peroxisomes is B-cell lymphoma 2 (BCL2)-antagonist killer (BAK) that previously was akin to outer mitochondrial membrane (OMM), but now belongs to peroxisomal membrane. The role of this protein is regulation of peroxisome’s permeability. Interorganelle crosstalk between peroxisome and mitochondria involves some pathways (e.g. PGC1ɑ and PPARγ), causing biogenesis promotion of both mitochondria and peroxisomes. Of course, ER and mitochondria involvement should be considered during peroxisomal proliferation. Moreover, mitochondrial-derived vesicles (MDVs) should be considered in the connection between mitochondria and peroxisome. Some of these MDVs target a small subset of peroxisomes, and the cargo of peroxisomal MDVs differ owning to lysosomal MDVs. Contents of lysosomal MDVs contribute to mitochondrial quality control. Other shared functions between mitochondria and peroxisomes are reactive oxygen and nitrogen species (ROS and RNS) generation through diffusion, redox-sensitive relationships, influence on signaling pathways, and contribution in lipid metabolism. Interorganelle crosstalk between mitochondria and lysosome is via information exchange, physical contact, and mitochondrial-derived vesicles. Any pathologic condition in these ways may contribute to disease states. One pathologic state in crosstalk between two organelles is destabilization of the lysosomal membrane that generates a crosstalk between lysosomes and mitochondrial vesicle traffic and promotes apoptosis.
So far, no similar systematic review and meta-analysis has been conducted on interorganelle crosstalk or communication. The twenty studies included in this review evaluated seven functional variables of mitochondrial crosstalk with other organelles. They contained impaired RC function, calcium homeostasis, apoptosis and autophagy, ROS levels, ATP levels, redox state, and lipid metabolism. In our research, the most important communication studies were related to mitochondria and peroxisome crosstalk (10%) via ROS overproduction (6.25%) by their negative effects on the cell. Moreover, overproduction of ROS in mitochondria leads to lipids, proteins, and mtDNA damage, which results in cell damage. RC dysfunction can induce an oxidative stress combined with high nitric oxide (NO) levels and may produce peroxynitrite. As such, 3.1% of studies were related to mentioned variable in mitochondria to nucleus crosstalk. Other variable of communication between mitochondria and nucleus organelles was related to decreased ATP levels and apoptosis (3.1%). Variable of calcium homeostasis has implications in interorganelle crosstalk between endoplasmic reticulum and mitochondria, that was assessed 60%. This crosstalk impacted Ca2+ homeostasis in 15.6% of studies. MCSs between these organelles enhance oxidative metabolism via activating pyruvate dehydrogenase and mitochondrial calcium levels for initiating apoptosis at physiologic levels. Other protein in facilitating Ca2+ fluxes into mitochondria is mitofusin-2 (30); in our study, these molecules were attributed to Mt-ER crosstalk in 16.6% of studies. It is worth noting that the most important result of this communication between two organelles was related to apoptosis (15.6%). Moreover, increased ROS levels and lipid metabolism (12.5% and 6.25%) were also involved in this crosstalk. Mt-PO crosstalk consisted 10% of original studies, and 6.25% of variables were related to enhanced ROS levels in this crosstalk. Furthermore, 3.1% of functions of this crosstalk were related to redox state. Interorganelle connection between mitochondria and lysosome in this research accounted for 20% of communications and process of apoptosis, the most conspicuous interaction between mitochondria and lysosomes, was assessed as 9.3%. Moreover, this crosstalk had major impacts on Ca2+ homeostasis (3.1%), increased ROS levels (3.1%), imbalance of redox state (3.1%), and lipid metabolism (3.1%). An example of this crosstalk was represented in Ghavami et al. (23). study which cell death occurred by S100A8/A9 in mitochondrial crosstalk with lysosomes through ROS production and process of BNIP3 factor. Finally, our study revealed risk of 1.5 and odds of 2 on aging and age-related disorders in interorganelle crosstalk between mitochondria and peroxisome or lysosome.
5.1. Interorganelle Crosstalk in Kidney Diseases
The kidney organ needs extreme energy amount to maintain the body's metabolism, plasma hemodynamics, electrolytes, and water homeostasis, nutrients reabsorption, and hormone secretion. In other words, it is only second to the heart in mitochondrial count and oxygen consumption to provide appropriate function of kidney cells (31). Although the majority of mitochondrial dysfunction occurs in nondiabetic kidney disease, recent studies suggest that mitochondrial dysfunctions in kidney cells are pathological mediators of diabetic kidney disease (32). An important point is that Mfn2 protein plays a role in mitochondrial fusion and acts as a protein in mitochondrial tethering to the endoplasmic reticulum, facilitating juxtaposition, and calcium fluxes between two organelles. Continuation of discussion about crosstalk between two organelles in kidney cells is interorganelle communication between mitochondria and nucleus that PGC-1a leads to mitochondrial biogenesis through certain genes, oxidative phosphorylation, and ß-oxidation of fatty acid (33). In our study, 3.3% of studies were related to renal disease, indicating that organellar damage can cause various kidney diseases. Results of the present study showed that in 33.3% of studies, mt-ER interactions lead to focal segmental glomerular sclerosis (FSGS). Moreover, mitochondria-nucleus interactions in the present study revealed aldosterone-induced podocyte injury through SIRT1/PGC-1ɑ pathway in 33.3% of studies. Organelle crosstalk between mitochondria and peroxisome resulted in diabetic nephropathy in 33.3% of studies. The main limitations of this research were small size of the studies and lack of access to software. As far as the researchers investigated, this research is the first report of interorganelle crosstalk in kidney cells.
6. Conclusions
Medical science promotes through seeking physiopathological mechanisms of diseases that determine patient's health and safety. Elucidating the mechanisms of disease not only results in novel diagnostic methods but also causes the establishment of appropriate therapeutic and preventive planning. Organelle crosstalk is conducted via both vesicular and non-vesicular transports and exchange their contents such as proteins, lipids, and ions for cell homeostasis. Therefore, recognition of intracellular organelles and communication between them can affect cell activity and function. Any disruption of such communication is associated with a variety of pathophysiological conditions such as metabolic disorders, cardiovascular disease, and cancer. Organellar dysfunction in kidney cells can affect other organelles and impacts certain pathologies such as kidney dysfunction and metabolic disease. Future meta-analyses are necessary for performing important scores of communication between these organelles in medicine.