1. Background
Malaria is an infectious disease in developing countries, which is characterized by chills (shivering), fever, and sometimes severe and fatal complications. It is globally important in terms of health, being one of the six important diseases in the World Health Organization (WHO) programs for tropical and subtropical regions (1, 2). According to the latest WHO report released in 2016, despite various malaria control and anti-malaria programs, approximately 216 million cases of malaria have been reported from 91 countries, which has been about 5 million cases more than the figure reported in 2015. The number of fatalities has been reported to have been 445,000 persons worldwide (3). Iran is one of the recognized malaria-endemic countries in the eastern Mediterranean region. The southern and southeastern regions of Iran are malaria-endemic, and the two southeastern provinces of Sistan and Baluchestan and Hormozgan, as well as Jiroft and Kohnuj counties in Kerman province, have the highest rate of malaria cases across the country (4, 5). The primary goal of the malaria elimination program on the Horizon of Iran 1404 (2025) is to stop the local transmission of the disease and turn Iran into a malaria-free region (6, 7).
The basic strategy in the global malaria control program is to control Anopheles mosquitoes, which has been proved as one of the best and most effective methods of stopping and reducing the malaria transmission rate around the world. The awareness of the composition of Anopheles species and the number of sporozoites in their salivary glands, which determines the role of each species in the transmission of the disease, are key epidemiological indicators required to plan for malaria control and prevention methods commensurate with local conditions (8, 9).
Various biochemical, molecular, and immunological methods, such as isoenzyme electrophoresis, polymerase chain reaction (PCR), and monoclonal antibodies, are commonly used to diagnose Plasmodium in mosquitoes (10, 11). One of the most common methods for detecting malaria parasites in Anopheles mosquitoes is the dissection of salivary glands in captured female Anopheles, using stereomicroscope and light microscopy. However, this method is quite laborious, time-consuming, and of a low sensitivity level (10, 12). Among many methods proposed as alternatives to the microscopic detection of parasites in mosquitoes, nested PCR using 4 primers for the 18s subunit ribosomal ribonucleic acid (18ssrRNA) gene was introduced as a sensitive method with high specificity for the detection of Plasmodium species (P. falciparum, P. vivax, P. malaria, and P. ovale) (13). The nested PCR assay is highly sensitive and highly specific in malaria detection, which is capable of detecting 1 - 10 parasites per microliter (µL) of blood and is five times stronger than the microscopic detection power of Giemsa-stained spreads. Apart from detecting parasite species, it is also capable of detecting mixed infections. In addition, the high sensitivity and specificity of this method indicate its importance in the diagnosis of low-parasite cases and asymptomatic malaria. The nested PCR is a very suitable method for the detection of malaria in regions of a low transmission rate, which could be used to monitor drug resistance. Many samples could also be tested concurrent with using this technique (10).
In southern and southeastern Iran, due to the relatively favorable climatic and geographical conditions as well as its proximity to Pakistan, malaria is a major public health problem. Sistan and Baluchestan province, in southeastern Iran, has a long common border with Pakistan and has the highest incidence of malaria (14).
2. Objectives
This study aimed to investigate the rate and type of the infection of female Anopheles mosquitos with sporozoites of the malaria parasite using the nested PCR in Chabahar and Konarak counties of Sistan and Baluchestan province.
3. Methods
3.1. The Study Region
Sistan and Baluchestan province is located in southeastern Iran, with Chabahar and Konarak counties located in the southeast of the province. Chabahar has three urban centers, including Chabahar, Negour, and Polan (Figure 1). It also has three districts, including the central district, Dashtiari, and Polan. In addition, it has six rural districts, including Pirsohrab, Kambel-e Soleyman, Nowbandian-e Payyn, Bahoukalat, Sand-e-Mirsuban, and Polan, with 446 inhabited villages. Konarak has two urban districts, including Konark and Zarabad, and four rural districts, namely Zarabad-e Sharqhi, Zarabad-e Gharbi (Caravan), Kahir, and Jahliyan. This province is located on the northern coast of the Oman Sea, where the warm and humid climate of the region is suitable for Anopheles species, which has made various Anopheles species be the main vector responsible for malaria transmission in southeastern Iran.
3.2. Sampling
This cross-sectional descriptive study was conducted in the second half of 2018 in the high-risk regions of malaria transmission in Sistan and Baluchestan province, i.e., Chabahar and Konarak. The disease in these regions has two peaks, from late April to early June and from August to late November, with the second peak being more severe. The districts surveyed in Chabahar included Daregas, Owraki, and Bahu Kalat. In addition, the regions studied in Konarak were Zarabad, Heyan, Islamabad, and Joholou (Figure 1). The villages selected were sampled once every 15 days, and a total of 360 blood-fed Anopheles mosquitoes were hunted manually from indoor places (humans and animals) as well as natural and artificial sites (pit-shelters) by suction or aspirator tubes. All specimens caught by aspirators were killed by small cotton pads placed on plastic cups. Just after the samples were collected, they were immersed in tubes containing 70% alcohol and transferred to medical centers in Chabahar and Konarak. Using high magnification entomology loops, the blood-fed Anopheles were isolated. Next, they were identified and separated using the Iranian Adult Anopheles Recognition Key (15). The separated Anopheles samples were transferred into 1.5-ml tubes, and after writing specifications on the tubes, they were stored at -20°C until DNA extraction.
3.3. DNA Extraction
DNA extraction was conducted using DyanBio (DyanBioTM Boold/Tissue DNA Extraction Mini Kit) according to the instructions. The solution under the DNA column was kept at -20°C until PCR assessment.
3.4. Nested PCR
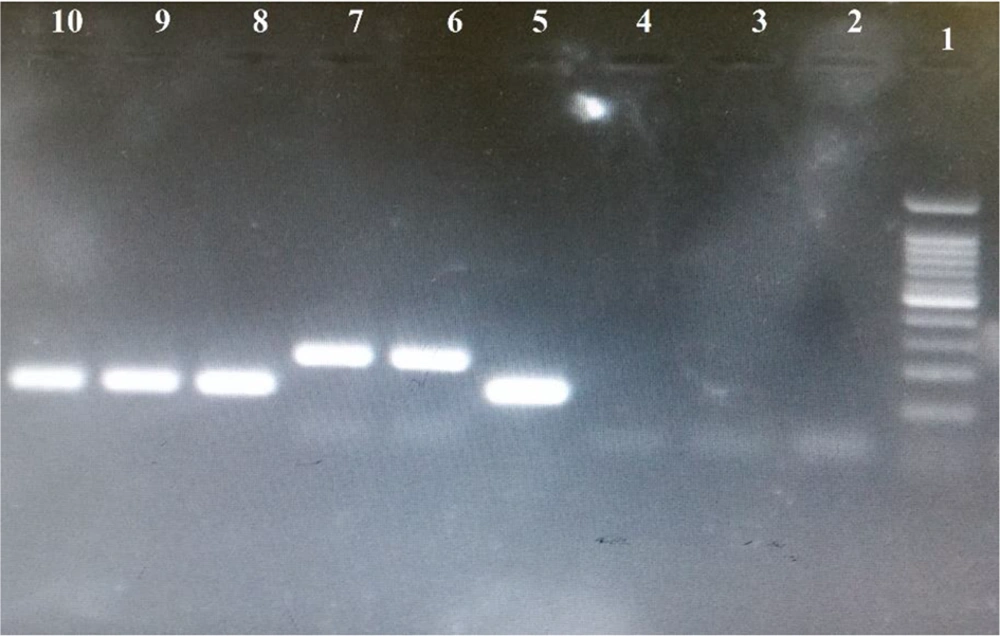
Nested PCR was performed with four pairs of primers and cycling conditions as described by Ebrahimzadeh et al. (16). The concentration of the final solution was 20 Pmol/μL. The primers were kept at -20°C. DNA samples were processed by PCR to amplify species-specific sequences of 18ssrRNA genes of P. vivax and P. falciparum. The first PCR utilized rplu 5 and rplu 6 primers, and the second PCR used rFAL.1, rFAL.2, rVIV.1 and rVIV.2 primers (17). PCR products of the second round of the PCR were loaded onto a 2% agarose gel, and the results were compared with standard band markers of P. vivax, 120 bp, and P. falciparum, 205 bp (Figure 2).
Electrophoresis of the 18ssrRNA gene nested PCR products on a 2% agarose gel. Lane 1: DNA marker 100 bp, Lane 2: Negative control, Lanes 3,4: Negative sample, Lane 5: control positive P. vivax (120 bp), Lane 6: control positive P. falciparum (205 bp), Lane 7: P. falciparum (205 bp), Lanes 8, 9 and 10: P. vivax.
4. Results
A total of 360 female Anopheles mosquitoes were captured. Of 200 samples collected from Daregas, Owraki, and Bahu Kalat in Chabahar, four An. stephensi (2%) were infected with P. vivax that positive cases were taken from Daregas (Two positive cases) and Bahu Kalat villages (Two positive cases). In addition, of 160 samples collected from the rural districts of Konarak, including Zarabad, Heyan, Islamabad, and Joholu, two An. stephensi (1.25%) were determined to be infected with P. falciparum that positive cases were taken from Zarabad (Table 1). In total, 1.66% of the vectors of An. stephensi were infected with the Plasmodium parasite.
| Sample Collection Areas | An. stephensi (%) | An. culicifacies (%) | An. d'thali (%) | An. polacrimus (%) | Total |
|---|---|---|---|---|---|
| Daregas | 50 (50) | 44 (44) | 5 (5) | 1 (1) | 100 |
| Bahu Kalat | 24 (48) | 17 (34) | 7 (14) | 2 (4) | 50 |
| Owraki | 33 (66) | 15 (30) | 2 (4) | 0 | 50 |
| Zarabad | 23 (40.35) | 23 (40.35) | 8 (14.03) | 3 (5.27) | 57 |
| Heyan | 15 (50) | 8 (26.67) | 7 (23.33) | 0 | 30 |
| Islamabad | 16 (45.71 | 13 (37.14) | 5 (14.3) | 1 (2.85) | 35 |
| Joholou | 28 (73.68) | 10 (23.62) | 0 | 0 | 38 |
5. Discussion
Malaria is still a major health and medical problem in southeastern Iran, especially in Sistan and Baluchestan province. It plays an important role in restricting the economic, social, and economic development of this region and wastes a great deal of capital, labor, time, and energy annually. In this study conducted in two malaria-endemic regions of Sistan and Baluchestan province (southeastern Iran), a total of 360 female Anopheles mosquitoes belonged to four species, including An. stephensi, An. culicifacies, An. fluriatilis, and An. d'thali were caught. In a study aimed at identifying malaria vectors in high-risk regions of malaria transmission in Hormozgan province (Iran), Azizi et al. (2011) reported that adult anopheles belonged to the eight species of An. stephensi, An. moghulensis, An. d'thali, sergeanti, An. culicifacies, An. fluviatilis, An. superpictus, and An. trochidae (18). A study conducted in Fars province (Iran) by Kalantari et al. (2017) showed that the 1,073 anopheles caught belonged to the four species of An. d'thali, An. superpictus, An. acharovi, and An. stephensi (19). Therefore, the diversity of Anopheles species in Iran is created by varied ecological, climatic, altitude, and atmospheric conditions of different geographical regions.
In the present study, 200 Anopheles mosquitoes collected from the regions studied in Chabahar were determined to be the dominant species of An. stephensi. In addition, An. culicifacies, An. polacrimus, and An. d’thali were among the other important species found in this area. According to the findings, four species of Anopheles mosquitoes (2%) caught from Daregas and Bahu Kalat villages were infected with P. vivax. This finding is further supported by the fact that the predominant malaria species in Iran is P. vivax (20), and that 2% of mosquitoes are infected in Chabahar. Similar studies (Mirahmadi et al., 2019; Asmar et al., 2005; Oshaghi et al., 2003) conducted in malaria-endemic regions in Iran have shown that the predominant species causing malaria is Anopheles P. vivax (21-23). In the same vein, Guo Yu et al. (2013) and Kyu Sik Chang et al. (2016) performed molecular analyses of malaria vectors in China and South Korea, respectively, and reported that the predominant type of P. vivax malaria and the dominant Anopheles malaria were minimus and kolini, respectively (24, 25).
In the present study, of 160 species collected from the studied regions of Konarak county, An. stephensi was the dominant species followed by An. culicifacies and An. d’thali as the second and third most dominant species, respectively. It was also found out that the two species of An. stephensi mosquitoes (1.25%) captured from Zarrabad village were infected with P. falciparum. This case of infection has probably been due to the illegal entry of Afghan and Pakistani immigrants, who have been working on banana, mango, lotus, and date farms, and have been traveling to Afghanistan and Pakistan as the countries with the highest risk of malaria transmission (21, 26). In addition, the studies conducted by Nejati et al. (2012) in the city of Konarak, Raeesi et al. (2009) in the endemic regions of Iran, and Podat et al. (2006) in Bandar Abbas showed that essential malaria changes and the increased incidence of the disease depend on the presence of aliens (26-28).
Results from the analysis of the variety of mosquito species showed that An. stephensi was the most frequent species in Chabahar and Konarak. The similar coastal climates of these two counties are the main reason for the similar distribution of this species. An. stephensi appears to be the major vector of malaria in urban, lowland, and coastal regions (29, 30).
A study by Kalantari et al. (2017) in Fars province, Iran showed that An. d'thali was the most frequent species, and no infection with Plasmodium parasite was reported in mosquitoes using nested PCR. In the present study, a total of 1.66% of An. stephensi were infected with the Plasmodium parasite because Sistan and Baluchestan province is one of the malaria-endemic regions. In addition, the inflection of An. stephensi with Plasmodium parasite could be attributed to the differences in the endemic and ecological conditions of this province with those of Fars province (19). The study by Gonzalez et al. (2017) in northern Kenya using nested PCR showed that Anopheles albuminous was the most frequent species, which was infected with P. vivax and P. falciparum (31). In the same vein, Nepomichene (2015) conducted a study in Madagascar using nested PCR assay and showed that 9.5% of An. castanei were infected with P. vivax and P. falciparum (32). Ashwani Kumar et al. (2016), in a study in western India using nested PCR assay, found out that 2.8% of An. subpictus were infected with P. vivax and P. falciparum (33). A study was conducted by Vu Duc Chinh et al. (2019) in Vietnam using nested PCR assay on infectious cases in captured Anopheles. According to the results of this study, Anopheles inui was the dominant species in the region infected with P. falciparum, P. vivax, P. cynomolgi, and P. knowlesi (34).
According to the results of the four studies reviewed above, the prevalence of different Plasmodium and Anopheles species in different regions of the world is affected by many factors, among which climate changes and ecological conditions are the most effective ones. Therefore, the results of the present study concerning the prevalence of Plasmodium and Anopheles species are different from those of the above-mentioned ones.
In this study, a nested PCR assay was used, which is more sensitive than the conventional PCR. In addition, apart from detecting parasite genus and species, it is capable of detecting mixed infections. The 18ssrRNA gene was used for this purpose. This nucleotide sequence is fixed in eukaryotic organisms, especially in the parts involved in the formation of the second structure of the molecule. Therefore, oligonucleotide primers specifically binding to the protected part of the 18ssrRNA of genus Plasmodium and also uniquely binding to a part of the 18ssrRNA gene of each Plasmodium parasite species could be developed and used for identifying Plasmodium species. The present study showed that nested PCR assay could be easily used for a large number of samples. This technique does not require the dissection of Anopheles’ salivary glands; in addition, dried and old specimens can be examined using this method. Therefore, there is no need for equipping laboratories and dispatching personnel to malaria-endemic regions; hence, specimens collected in the region could be analyzed and tested in central laboratories (23, 30).
According to epidemiological studies, Iranian malaria species, unlike those in African and other countries, vary according to climatic conditions and are almost eliminated (17, 35). Since the malaria-endemic regions of Iran are undergoing the process of malaria elimination, one of the most effective ways of monitoring the above-mentioned program is to monitor the infection status in female Anopheles mosquitoes. In contrast to the past studies, the results of the present study showed that there are some infection cases in malaria-carrying mosquitoes in Chabahar and Konarak, so it seems that the elimination of malaria by 2026 will require further studies, investigations, and arrangements.
In the present study, the species infected with the Plasmodium parasite was the An. stephensi mosquitoes, which is in contrast to other studies in the southeastern region. This may be due to annual spraying (twice a year) of mosquito, problems with mosquito trapping, and malaria elimination program in Iran. Also, An. stephensi has a higher chance of coming into contact with humans due to being domesticated, and their presence in domestic and foreign places is considered a warning sign for the outbreak of malaria. On the other hand, the vector control program is planned based on the duration of seasonal activity of Anopheles mosquitoes and the trend of population changes (36, 37).
Pakistan is adjacent to Iran's Sistan, and Baluchestan Province, which according to the WHO, is one of the six countries in the Eastern Mediterranean region with a high rate of malaria transmission and about 100% of the population lives at risk, and the majority of malaria species are imported from Iran. Studies in Pakistan in 2018 show that 24% of malaria falciparum and 10% mixed infection by these two species P. falciparum and P. vivax, and may be due to the contrast between the percentage of infection reported in the carrier and the prevailing percentage of P. vivax in Iran (38).
5.1. Conclusions
The present study showed that the species infected with Plasmodium parasites in the studied regions was An. stephensi, which is the most frequent species among other species. The nested PCR may be a better method for detecting Plasmodium parasites in malaria vectors than other diagnostic methods.