1. Background
Tacrolimus is a calcineurin inhibitor, which is an immunosuppressive drug, widely used to avoid tissue transplantation rejection and treat autoimmune diseases (1). Few studies analyzed the effects of tacrolimus on the male reproductive system; some indicate that tacrolimus can cause testicular weight loss, loss of epithelial thickness in seminiferous tubules (2, 3), decrease in the number of spermatocytes, and increase in the number of spermatids and spermatozoa with damaged heads and tails (3). According to evidence, the activation of the renin-angiotensin system (RAS) and inflammation can exacerbate the toxic effects of tacrolimus. In fact, sodium depletion activates RAS in a cascade way that may increase tacrolimus toxicity in the cardiovascular system in experimental models (4). Numerous tissues need RAS for activity. Evidence proves the presence of local renin-angiotensin in testicle, prostate, vas deferens, and seminal fluid; however, the role of RAS is still undetermined and needs more clarifications. There is also evidence that the local RAS affects tubular constriction, spermatogenesis, maturation of sperms, capacitation, acrosome exocytosis, and zygosis. There are several sites for the local RAS to interfere with the male reproductive system (5, 6). Hormones produced by testicles play a pivotal role in adjusting local RAS elements (7). Results indicate that local RAS has a subsidiary role in fertilization. This finding can be explained by the existence of blood-testis barrier and the drug entrance blocking effect of this barrier (5). The ACE2 is a regulatory enzyme in steroidogenesis, which exists in the ovary and testicle. It affects the reproductive system and the activity of germ cells (8). In the male reproductive system, ACE2 is selectively expressed by adult Leydig cells in testicles and produces angiotensinogen (1-7). Studies indicated that males with low serum levels of ACE2 confront severe disorders in spermatogenesis and reproductive system function (9).
2. Objectives
The present study aimed at determining the toxic effects of tacrolimus on testis and evaluating the blocking effect of captopril on angiotensin-converting enzyme (ACE) and the blocking effect of losartan on angiotensin receptors.
3. Methods
A total of 36 adult male Wistar rats (weighing 200 ± 20 g) was provided by the Central Research Laboratory in Dezful University of Medical Sciences and assigned to six groups (n = 6). The rats were kept under standard conditions (a temperature of 25°C and a 12-12: hour light-dark cycle) for 30 days (10). The study protocol was approved by the Ethics Committee of Dezful University of Medical Sciences (ethical code: IR.DUMS.RFC.1396.25), and all the experiments were performed in accordance with the guidelines for the safe handling of animals.
3.1. Animal Grouping
The first group, controls, received normal saline. The second group received a daily dose of 1 mg/kg tacrolimus (Tac) via intraperitoneal injections (i.p.i.). The third group received a daily dose of 1 mg/kg tacrolimus (i.p.i.) and 100 mg/kg captopril (Tac + Cap) through gavage (11). The fourth group received daily doses of 1 mg/kg tacrolimus (i.p.i.) and 80 mg/kg losartan (Tac + Los) through gavage (12). The fifth group received a daily dose of 80 mg/kg losartan (Los) through gavage, and the sixth group a daily dose of 100 mg/kg captopril (Cap) through gavage (13).
3.2. Sample Collection
The rats were anesthetized using chloroform and euthanized by bleeding from the heart. The serum samples were collected to measure the levels of testosterone and ACE2. Testosterone was measured by ELISA kits (Rat) (OKCA00179), based on the competitive enzyme-linked immunosorbent assay technique. ACE2 levels were measured by sandwich immunoassay technique with the rat angiotensin converting enzyme 2 (ACE2) ELISA kit (catalogue number: MBS 700969). Both testicles were fixed in 10% formalin buffer after dissection of overweight tissue. Eventually, the serial 5-6-µM testicle slides were prepared following the sectioning guidelines and stained with hematoxylin-eosin. Five slides were provided from each testicle in each group. Morphological and histopathological examinations included spermatogonia, primary spermatocytes, spermatids, and Sertoli and Leydig cells number. The epithelial thickness of seminiferous tubule was measured using a Dino-Lite Digital Microscope and the related software.
3.3. Statistical Analysis
All the analyses were performed using SPSS version 16 (SPSS Inc., Chicago, Ill., USA). The within-group variation was analyzed by one-way analysis of variance. The Fisher least significant difference test was used to evaluate significant differences between the groups. P ≤ 0.05 was considered statistically significant.
4. Results
Based on the obtained results, as shown in Table 1, testosterone reduced significantly in Tac rats, compared with the control group (P ≤ 0.05).
| Group | Testosterone (ng. dL-1) | ACE2 (U.L-1) |
|---|---|---|
| Control | 10.25 ± 3.75 A | 6.64 ± 3.53A |
| Tac | 4.44 ± 5.21C | 4.43 ± 2.91B |
| Tac + Cap | 9.35 ± 3.9A | 3.71 ± 3.67B |
| Tac + Los | 6.98 ± 4.56 B | 4.2 ± 3.18B |
| Los | 10.35 ± 4.11A | 5.98 ± 4.3A |
| Cap | 11.34 ± 3.77 A | 5.23 ± 4.1A |
Mean Levels of Testosterone and ACE2 in Rats a
The Tac + Cap, Cap, and Los groups were not significantly different from the control one in testosterone level (P > 0.05). However, the Tac + Los group had a significant reduction in testosterone level (P ≤ 0.05).
The Tac, Tac + Cap, and Tac + Los groups faced a decrease in ACE2 level, compared to the control group (P ≤ 0.05). The ACE2 level maintained unchanged in the Los and Cap groups, compared with controls (P > 0.05).
As shown in Table 2, the number of spermatogonia in all groups was not significantly different from those of the control group (P > 0.05). The number of primary spermatocytes and Leydig cells significantly reduced in Tac rats, compared with controls (P ≤ 0.05).
| Group | Spermatogonia | Primary Spermatocyte | Spermatid | Sertoli Cells | Leydig Cells |
|---|---|---|---|---|---|
| Control | 15.41 ± 3.12A | 13.51 ± 4.47A | 21.35 ± 3.93A | 8.24 ± 4.17A | 18.35 ± 4.17A |
| Tac | 13.24 ± 5.52A | 8.25 ± 3.44C | 11.36 ± 5.36B | 4.76 ± 3.45B | 7.47 ± 3.61C |
| Tac + Cap | 13.66 ± 4.25A | 13.12 ± 4.29A | 13.24 ± 6.11B | 4.28 ± 4.61B | 17.52 ± 4.97A |
| Tac + Los | 13.67 ± 4.33A | 10.94 ± 5.13B | 14.65 ± 3.77B | 3.74 ± 3.64B | 12.29 ± 4.94B |
| Los | 15.77 ± 4.26A | 14.33 ± 3.8A | 20.67 ± 4.3A | 9.72 ± 4.35A | 17.47 ± 4.52A |
| Cap | 14.36 ± 3.78A | 13.42 ± 3.27A | 24.33 ± 4.91A | 8.46 ± 4.19A | 19.17 ± 3.74A |
Means of Spermatogonia, Primary Spermatocyte, Spermatids, and Sertoli and Leydig Cell Number in Rats a
There was no significant difference in the number of primary spermatocytes and Leydig cells between the Tac + Cap and control groups, even though it was significantly higher than that of the Tac group (P ≤ 0.05). The number of primary spermatocytes and Leydig cells descended and elevated in Tac + Los and Tac groups, respectively, compared with the control group. The number of spermatids and Sertoli cells reduced significantly (P ≤ 0.05) in the Tac, Tac + Los, and Tac + Cap groups compared to controls. In comparison with the control group, no significant difference was observed in average testicle weight among different groups (P > 0.05) (Table 3).
| Group | Testis Weight | Seminiferous Diameter | Epithelium Thickness |
|---|---|---|---|
| Control | 1.54 ± 3.42A | 196.24 ± 3.42A | 68.71 ± 5.36A |
| Tac | 1.34 ± 3.61A | 127.21 ± 3.21C | 30.28 ± 6.35C |
| Tac + Cap | 1.39 ± 3.72A | 187.34 ± 4.21A | 65.29 ± 3.61A |
| Tac + Los | 1.42 ± 3.22A | 145.71 ± 4.17B | 45.78 ± 4.12B |
| Los | 1.62 ± 6.41A | 192.38 ± 4.23A | 70.73 ± 3.73A |
| Cap | 1.47 ± 3.66A | 200.31 ± 4.26A | 67.32 ± 3.99A |
Means of Testis Weight, Diameter of Seminiferous Tubule, and Epithelial Thickness in Different Groups a
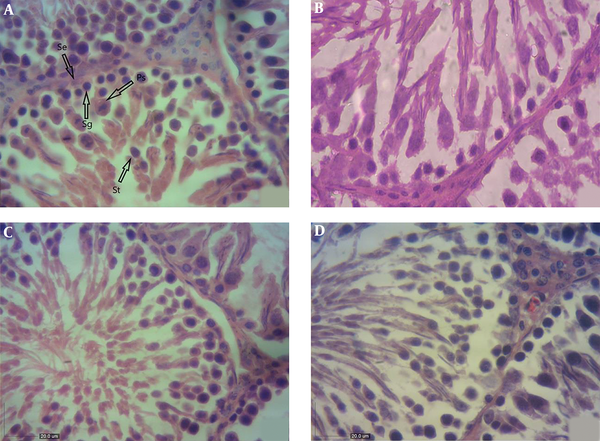
As shown in Table 3, in comparison with the control group, a significant loss was observed in the epithelial width of seminiferous tubules in Tac rats (P ≤ 0.05). The Tac + Cap and Tac + Los groups had a thicker epithelial layer in seminiferous tubules compared to that of the Tac group, while no significant difference was observed with the control group. The epithelial thickness of seminiferous tubules was significantly lower in the Tac + Los group than controls and remarkably higher than that of the Tac group (Figure 1A - D).
A, Light photomicrographs of the testis in the control group (H&E, X400); the shape of seminiferous tubules and the height of the epithelial layer are normal. Arrows: Ps: primary spermatocyte; Sg: spermatogonia; St: spermatid; Se: Sertoli cells. B, Light photomicrographs of the testis in the tacrolimus-received group (H&E, X400); the seminiferous tubules have an irregular shape and the epithelium is desultory. The number of primary spermatocytes and Leydig cells reduced. C, Light photomicrographs of the testis in the tacrolimus and captopril receiving group (H&E, X400); the shape of seminiferous tubules and the height of the epithelial layer are normal. D, Light photomicrographs of the testis in the tacrolimus and losartan receiving group (H&E, X400); the shape of seminiferous tubules is normal and their epithelial layer is thick.
5. Discussion
Tacrolimus is an immunosuppressive drug with a macrolide structure used as an immune system suppressor in order to lower the chance of rejection for organ transplantation. Tacrolimus has a direct effect on the reproductive system; however, very few studies focused on it. Some studies indicated testicle weight loss (2), loss of epithelial thickness in seminiferous tubules (3, 14, 15), decrease in the number of spermatocytes, increase in the number of damaged spermatozoa, dissection of heads and tails in spermatozoa, and abnormal maturation of the flagella (16). The present study aimed at evaluating the role of RAS, the effect of its products on testicles, the toxic effects of tacrolimus on testicle tissue structure, the blocking effect of captopril on ACE, and the blocking effect of losartan on angiotensin receptors as neutralizing pharmacological strategies for the toxic effect of tacrolimus. During the study, testosterone reduced significantly in rats merely receiving tacrolimus in comparison with the control group. A study by Tai et al. (17), indicated that the main testosterone product and the response of Leydig cells to human chorionic gonadotropin (hCG) stimulation did not significantly alter in tacrolimus-receiving rats. The present study results were inconsistent with those of the research by Tai et al (17). In the current study, the group receiving captopril along with tacrolimus had significantly higher levels of testosterone compared with those merely receiving tacrolimus. This elevation in hormone level can be due to increase in the number of Leydig cells following the use of captopril along with tacrolimus. Serum ACE2 level decreased in the tacrolimus-receiving group, and simultaneously receiving aptopril or losartan with tacrolimus could not compensate for the serum level of ACE2. The number of spermatogonia was not significantly different between the tacrolimus and control groups. It was likely because these cells are produced in the embryonic period and, therefore, were not exposed to the drug. However, according to a study by Caneguim et al. (11), the groups that underwent tacrolimus treatment had a decreased number of spermatogonia due to apoptosis. This result did not correspond with those of the current study. In the present study, the rats that merely received tacrolimus had a decreased number of primary spermatocytes in comparison with controls. Caneguim et al. indicated a decrease in the number of primary spermatocytes while receiving tacrolimus, which correspond with the present study results (11). In the current study, the number of spermatocytes in the captopril-tacrolimus group was higher than that of the tacrolimus group but not as high as controls. Bechara et al. suggested that in tacrolimus-receiving animal models, high blood pressure was responsible for the lower spermatocyte production, and enalapril treatment can preserve the testicles from these alterations and retrieve natural spermatozoid production (18). These results corresponded with the current study findings in which a significant increase in the number of spermatocytes was observed in the Tac + Los group, compared with the Tac group. The number of spermatids was significantly lower in those merely receiving tacrolimus than controls. However, the Tac + Cap and Tac + Los groups were not significantly different from the Tac group, and the number of spermatids was low in these groups. In the study by Canegium et al., morphological examinations indicated a decrease in the number of spermatids in the tacrolimus-treated groups, consistent with the findings of the current study (11). The study by Masuda et al., reported alterations in seminiferous tubules, destruction of round spermatids and sperms, abnormal remnant bodies, and various malformations (3). In agreement with the current study results, their findings suggested that calcineurin drugs directly harm cellular growth and spermatogenesis. The number of Sertoli cells was significantly lower in the Tac group compared to controls. Simultaneous prescription of captopril and losartan, along with tacrolimus, could not restore these cells. According to the study by Canegium et al., tacrolimus-treated groups had a decrease in the number of germ cells due to apoptosis (11). They also suggested that, in addition to germ cells, tacrolimus affected Sertoli cells. The morphological findings of their study indicated a decrease in the number of Sertoli cells, inconsistent with the current study results.
In the present study, the rats merely receiving tacrolimus had a significantly fewer number of Leydig cells, compared with controls. Tai et al. studied the effect of FK506 (tacrolimus) on the function of Leydig cells in rats (17). The results suggested histologically normal Leydig cells in animals treated with tacrolimus. The Leydig cells are the main product of testosterone, and response to hCG stimulation in rats exposed to tacrolimus did not significantly differ in laboratory conditions. This finding did not match with the current study results. According to the present study, receiving captopril and losartan simultaneously with tacrolimus could increase Leydig cell number in rats. In the present study, the rats merely receiving tacrolimus had a thinner epithelial layer in seminiferous tubule, compared with controls. Canegiun et al. observed unorganized cellular layers in seminiferous tubules. The morphological findings of their study indicated a significant reduction in the epithelial layer of seminiferous tubules, consistent with the results of the current study (11). In the present study, the rats that merely received tacrolimus had a thinner epithelial layer in seminiferous tubule compared with controls. Canegiun et al. suggested that unorganized cellular layers are seminiferous tubules. The morphological results of the study was indicative of a significant reduction in the epithelial layer of seminiferous tubules, consistent with the results of the current study (11). Receiving captopril and losartan along with tacrolimus could lower the destructive effects of tacrolimus, although such effects were never totally neutralized.
5.1. Conclusions
Based on the current study results, tacrolimus decreases ACE2 level, is responsible for testicular toxicity emerging by destructive effects on the testicle tissue, and decreases testosterone level. Simultaneous prescription of captopril and losartan could somehow lead to the recovery of testicle tissue and increased testosterone level, but not to improve ACE2 level; therefore, the recovery of testicle tissue may be due to ACE2 approach or anti-inflammatory effect of captopril.