1. Background
Focal distal demyelinating neuropathy of median nerve, also called carpal tunnel syndrome (CTS), is the most common entrapment neuropathy in the upper limb (1). Several factors, including repetitive trauma, pregnancy, and systemic disorders such as rheumatoid arthritis (RA) and diabetes mellitus, can increase the risk of CTS (1-3). Patients diagnosed with CTS, usually present with numbness, paresthesia, pain in fingers or palm, and less often with weakness and muscle atrophy in advanced stages (2, 3). The diagnosis of CTS is made on the basis of clinical examinations and is conventionally confirmed by electrodiagnostic (EDX) findings (4-9). Moreover, the results of nerve conduction studies (NCS), as the main part of EDX testing, are used to classify symptom severities as mild, moderate, and severe grades (10).
Although different therapeutic options are available, the management of CTS symptoms is still challenging (5). Different conservative treatments used for mild to moderate grades of CTS, including topical application of non-steroidal anti-inflammatory drugs (NSAIDs), wrist splinting, physical modalities, and the local injection of corticosteroids (5-12). Reports reveal that many supportive therapies possess a short-term efficacy (13, 14). Surgical decompression is an effective treatment for CTS (15) in good candidates. In fact, it is approved that releasing the retinaculum through operative intervention is useful for moderate to severe grades (5). Despite obvious advantages, there are some concerns associated with the application of conservative treatments. It is observed that even steroid injections are occasionally associated with fat atrophy, skin depigmentation, or elevated blood glucose in patients with diabetes (16). Likewise, the prolonged use of NSAIDs causes gastrointestinal symptoms, renal failure, etc. (5).
Topical medications are relatively safe due to their mechanism of action (17, 18). In the past decades, a huge number of studies were conducted to explore the therapeutic efficacy of different traditional herbal derivatives with a scientific approach. The use of herbal combinations has been common in the ancient Middle-East region. Among the most prevalent topical medications, some herbal oleogels, such as Boswellia (Frankincense) compounds recently gained more attention, especially for the management of different inflammatory conditions (19-23). There are many types of Boswellia species, and the most prevalent ones are B. serrata, B. carterii, etc. (24).
Earlier studies proved that Boswellia carterii Birdw contains alpha and beta Boswellic acids (25, 26). Also, the 3-O-acetyl-11-ketobeta-boswellic acid (AKBA) can reduce leukotriene synthesis and inhibit 5-lipoxygenase enzyme, hindering the production of pro-inflammatory enzymes (27). Additionally, B. carterii (BC) can efficiently suppress other inflammatory pathways, such as cyclooxygenase and complement cascade (24). According to reports, the BC extract contains 65% - 85% resin (a mixture of trepans), 21% - 22% gum (a combination of polysaccharides), and 5% - 9% essential oils (28). In this regard, promising results were obtained by using BC for osteoarthritis and RA management (24, 27, 29-32).
Several studies in recent years showed that BC can be used and investigated as an anti-inflammatory, anti-arthritis, and analgesic agent (31, 33-36). Moreover, the anti-hyperglycemic effect of a similar herb from the same family (B. serrata) is recently studied in patients with diabetes (37-39). It is noteworthy that neither local, nor systemic toxicity is detected so far (31, 33-36, 38, 39). Although available studies support the anti-inflammatory and pain relieving effects of BC, its therapeutic effect on neuropathic pain syndromes, such as carpal tunnel syndrome (CTS), needs to be investigated.
2. Objectives
Hence, the present study aimed at evaluating the efficacy of a topical BC formulation in pain relief and functional improvement among patients with CTS.
3. Methods
3.1. Participants
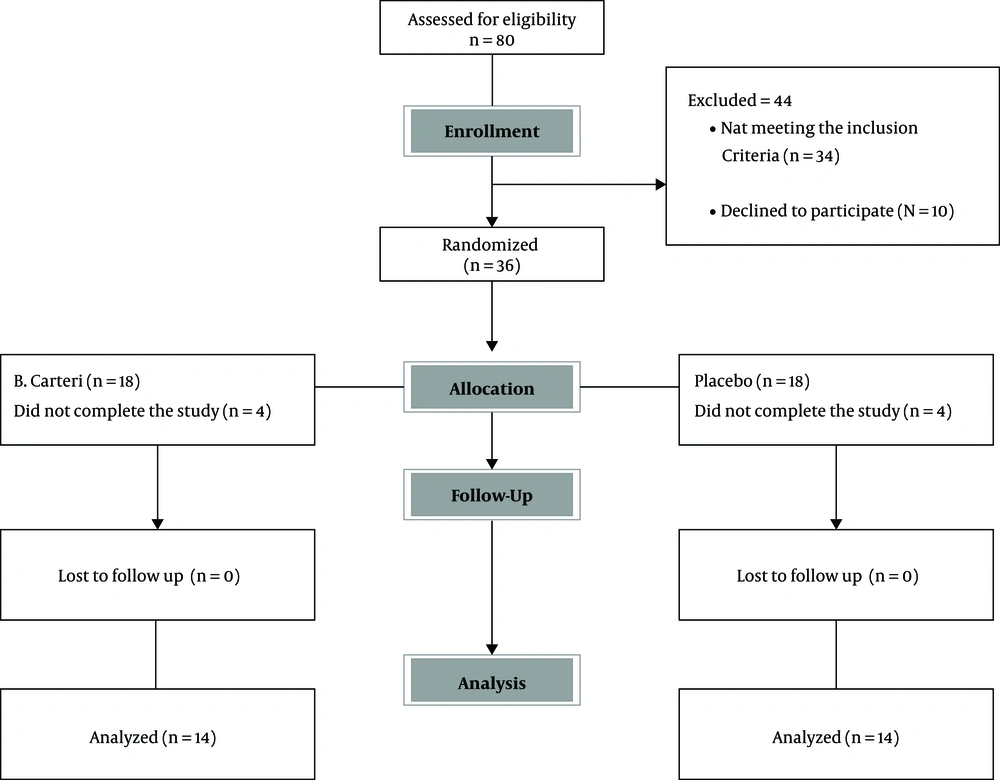
The current randomized, clinical trial (RCT) was conducted in Imam Khomeini Hospital Complex, the largest educational medical center of Tehran University of Medical Sciences (TUMS) Since August 2018 until the end of 2018. The study population was selected from 350 patients referring to the EDX clinic, among which, 80 with CTS symptoms were enrolled (Figure 1). Based on the inclusion and exclusion criteria, a total of 36 subjects with 72 hands were found eligible via electrodiagnosis results confirming the diagnosis of bilateral CTS (10, 40); finally, 28 patients completed the study.
3.2. The Inclusion and Exclusion Criteria
Only patients within the age range of 18 - 65 years with mild or moderate grades of CTS and symptoms lasting for longer than three months were included. The patients meeting the exclusion criteria as follows were excluded from the study: having severe CTS, a history of trauma, fracture, or surgery at the affected wrist, a history of treatment with steroid injections or splinting for CTS during the last six months, concomitant cervical radiculopathy or other similar conditions in EDX, which might interfere with symptoms, and a history of underlying diseases, such as multiple sclerosis (MS), polyneuropathies, RA, collagen vascular disease, hyperthyroidism, diabetes mellitus, or renal failure. Additionally, since BC could potentially stimulate the immune system, it should be avoided in those with any autoimmune diseases, such as RA, systemic lupus erythematosus (SLE), MS, and inflammatory bowel disease (IBD) (24). Therefore, patients with such diseases were also excluded.
3.3. Ethical Considerations
The current study protocol was approved by the Ethics Committee of Tehran University of Medical Sciences (ethical code: IR.TUMS.MEDICINE.REC.1396.4487). The study protocol was registered in the Iranian Registry of Clinical Trials (registration no.: IRCT20180731040647N1). The study protocol was conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a prior approval by the institution's human research committee. Also, all the patients were informed about the study objectives and signed a written informed consent form.
3.4. Formulation of Boswellia carterii
BC exudates were purchased from a traditional herbal shop (Attari) in Tehran, Iran. Then, it was identified by a herbalist in Herbarium Center of School of Pharmacy at TUMS with the code number of PMP-897 [Boswellia sacra Flueck, Burseraceae]. Then, it was cleaned, powdered, and dissolved in sesame oil (ratio 1:4) in water bath (Bain Marie). Then, it was filtered and converted to oleogel by adding colloidal silicon dioxide (11% v/w). Also, placebo was made by liquid paraffin (Merck) and BC oil (5% v/v; to be similar to the main product in odor and color) was converted to oleogel by adding colloidal silicon dioxide (10% v/w).
3.5. Interventions
Demographic characteristics of the patients, as well as the baseline level of anthropometric and clinical variables, such as wrist circumference, body mass index (BMI), chronicity, and the severity grade of the disease, were recorded at the study onset. Initially, a 10-score Visual Analog scale (VAS) was used to measure the pain intensity; in which 0 showed no pain while 10 indicated the maximal pain a patient could tolerate. A Persian version of Boston CTS questionnaire (BQ) was also used as a primary tool ti measure the clinical outcomes (41). This method comprised of two independent sections: Symptoms Severity scale (SSS) (11 items) and Functional Status scale (FSS) (eight items). All the 19 items are scored based on a five-point Likert scale where the higher scores indicate more severe conditions. However, the NCS parameters and pain-free grip-strength (PFGS) were evaluated as the secondary outcome measuring tools. PFGS was measured per kilogram in the affected hand, utilizing a grip dynamometer. The measurements were repeated three times and the maximum value was recorded as the final PFGS score.
The main two waves in NCS were compound motor action potential (CMAP), and sensory nerve action potential (SNAP), measured by an experienced physiatrist (SZ.ER) using a Medelec® machine. All parameters, including the latency and amplitude of SNAP/CMAP, were measured according to the American Association of Neuromuscular and Electrodiagnostic Medicine (AANEM) standards (40). Moreover, the assessment of median CMAP was performed by recording on abductor pollicis brevis (APB) muscle. Besides, a famous neurophysiological scale was used as a grading system (10). The criteria to diagnose mild CTS were SNAP latency > 3.6 ms with preserved SNAP amp, normal CMAP amp, and latency, while the criteria to diagnose the moderate grade of CTS were SNAP latency of > 3.6 ms and CMAP latency of 4.2 - 6.5 ms with a preservation of SNAP (10).
Then, participants were randomly divided into two equal groups using a mobile-based random-number generator (n = 18 in each group). Additionally, numbered opaque envelopes were used to conceal random sequence. A resting wrist volar-splint was used during the night in both groups for six weeks. In addition to wrist splinting, the participants of either groups received a 50-g bottle of a topical oleogel: in one group BC oleogel was used and in the other one, the placebo oleogel. Both of them were consumed at a regular basis of 1.5 fingertips every 12 hours. To ensure the patients’ adherence to the protocol during this period, they were under observation through regular weekly telephone calls. Also, use of volar-splint was instructed again.
This investigation was a triple-blinded RCT, in which the patients, the clinical assessors, and the person who performed data analysis were blind to the type of treatment. Patients in both groups were evaluated by a senior PM & R resident, and reassessed at 8th and 12th weeks of follow-up, to record their improvement in VAS, PFGS, and two parts of BQ. Also, changes in EDX parameters were checked by a senior physiatrist at all visits. Moreover, the related minor or major complications were also evaluated during the follow-up period.
3.6. Data Analysis
The descriptive results were presented as tables for qualitative variables, while central-dispersion indicators as mean ± standard deviation (SD) were used for quantitative variables. Data were analyzed with SPSS version 24 (SPSS Inc., Chicago, IL, USA). The Kolmogorov-Smirnov test was used to check the normality in distribution. To compare the differences between the two groups, the Chi-square and Student t- tests were applied for categorical and continuous variables, respectively. Also, the repeated measures method was used to reveal intragroup changes. In the current study, P-value < 0.05 was considered as the level of significance.
4. Results
Totally, 36 patients were randomly assigned into two groups. Among them, eight subjects did not complete the study due to the following reasons: three patients moved to other cities and were out of reach; two individuals had personal reasons (a relative passed away); one person did not answer any of his contact numbers; and two cases did not apply the medication regularly. Thus, 28 individuals completed the treatment, among which fourteen patients received topical oleogel of Boswellia, while the other 14 subjects received placebo. All patients had almost the same baseline clinical characteristics and were relatively comparable regarding their severity grades and anthropometric variables (Table 1). As previously denoted, the olegel usage lasted six weeks.
| Variable | Placebo (N = 14) | Boswellia carterii (N = 14) | P-Value |
|---|---|---|---|
| Age, y | 43.64 ± 7.8 | 47.58 ± 11.4 | 0.30 |
| Gender | 0.25 | ||
| Male | 4 (29) | 3 (22) | |
| Female | 10 (71) | 11 (78) | |
| Height, cm | 163.8 ± 17.0 | 168.8 ± 13.4 | 0.39 |
| Weight, kg | 82.0 ± 6.4 | 80.43 ± 11.4 | 0.95 |
| Severity grade | 9: 5 | 10: 4 | 0.29 |
| Mild (%): Moderate (%) | (64: 36) | (71: 29) | |
| VAS baseline | 4.07 ± 2.7 | 5.73 ± 2.9 | 0.13 |
| SSS-BQ baseline | 23.60 ± 8.9 | 29.53 ± 9.4 | 0.1 |
| FSS-BQ baseline | 14.27 ± 5.8 | 18.35 ± 6.1 | 0.09 |
| PFGS baseline | 7.35 ± 3.0 | 6.9 ± 2.6 | 0.67 |
Abbreviation: SD, standard deviation.
aValues are expressed as the median (range) or mean ± SD or No. (%).
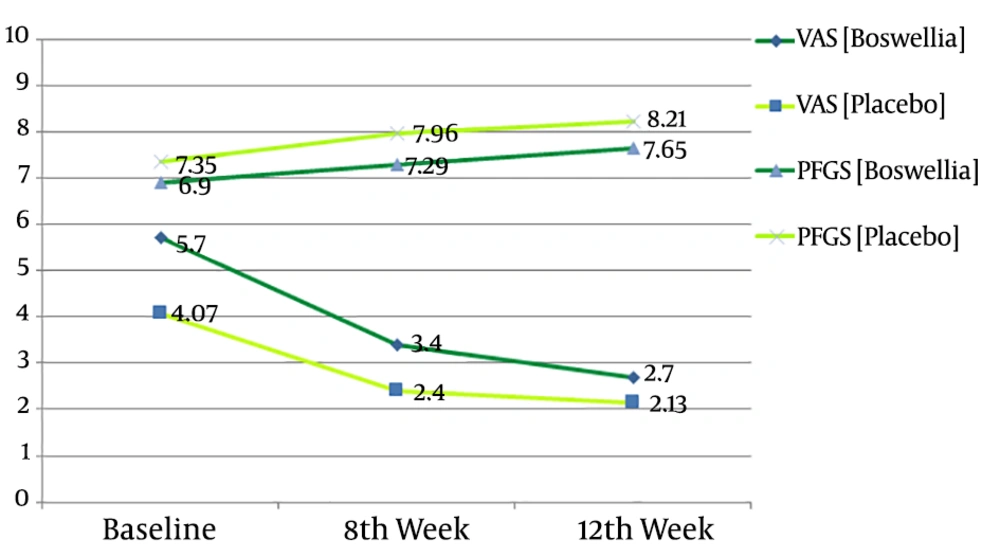
Based on the current study findings (Table 2), all clinical variables including pain based on VAS, grip strength, symptom severity, and functional status according to Boston questionnaire significantly improved in both groups across the two time-points (Figure 2). The pain intensity (VAS) significantly improved at weeks 8th and 12th in both groups. However, the amount of changes was greater at 8th week than 12th week, in favor of the B. Carteri group (MD: 1.0; P = 0.02 and MD: 0.57; P = 0.1, respectively).
| Group | Comparison | |||||||
|---|---|---|---|---|---|---|---|---|
| BQ-SSS | BQ-FSS | VAS | PFGS | SNAP Amplitude | CMAP Amplitude | SNAP Latency | CMAP Latency | |
| Placebo, mean ± SD | ||||||||
| Pre-intervention | 23.60 ± 8.9 | 14.27 ± 5.8 | 4.07 ± 2.7 | 7.35 ± 3 | 35.75 ± 10.7 | 9.96 ± 3.1 | 4.18 ± 0.4 | 4.55 ± 0.7 |
| 8th weeks | 17.13 ± 6.8 | 12.33 ± 3.44 | 2.4 ± 1.1 | 7.96 ± 2.8 | 38.71 ± 11.2 | 11.41 ± 4.3 | 4.20 ± 0.5 | 4.51 ± 0.8 |
| 12th weeks | 16.40 ± 5.6 | 11.67 ± 2.38 | 2.13 ± 1.9 | 8.21 ± 2.9 | 37.9 ± 11.0 | 10.99 ± 3.8 | 4.19 ± 0.4 | 4.32 ± 0.7 |
| P-valuea | 0.003 | 0.01 | 0.004 | 0.02 | 0.31 | 0.37 | 0.80 | 0.65 |
| Boswellia carterii, mean ± SD | ||||||||
| Pre-intervention | 29.53 ± 9.4 | 18.35 ± 6.1 | 5.7 ± 2.9 | 6.9 ± 2.6 | 34.94 ± 9.9 | 10.59 ± 3.3 | 4.15 ± 0.4 | 4.66 ± 0.8 |
| 8th week | 20.93 ± 6.4 | 15.07 ± 7.0 | 3.4 ± 1.5 | 7.29 ± 1.7 | 36.26 ± 10.8 | 10.32 ± 3.9 | 4.16 ± 0.4 | 4.67 ± 0.8 |
| 12th week | 18.80 ± 6.8 | 12.93 ± 3.79 | 2.7 ± 2.1 | 7.65 ± 1.8 | 34.81 ± 9.7 | 11.99 ± 4.0 | 4.21 ± 0.5 | 4.4 ± 0.7 |
| P-valuea | 0.001 | 0.007 | 0.001 | 0.01 | 0.34 | 0.29 | 0.73 | 0.61 |
| Between Groups Comparison | ||||||||
| P-valueb, MD | 0.001 (3.8) | 0.04 (2.74) | 0.02 (1.0) | 0.3 (0.67) | 0.3 (2.4) | 0.4 (1.1) | 0.5 (0.04) | 0.6 (0.16) |
| P-valuec, MD | 0.01 (2.4) | 0.06 (1.26) | 0.1 (0.57) | 0.4 (0.56) | 0.3 (3.1) | 0.4 (1.0) | 0.8 (0.02) | 0.7 (0.08) |
aChanges over time within the groups, based on repeated measure test.
bComparison between the two groups based on MD in 8th f/u using student t-test.
cComparison between the two groups based on MD in 12th weeks f/u using student t-test. Significant values are in bold format.
According to the results of Boston questionnaire, mean FSS score significantly decreased in both groups After 12 weeks follow-up. In the Experimental group the changes remained until the 12th week, while the placebo effect was only observed in the first visit and did not further change remarkably from Week 8 to week 12 (Figure 3). Again, the amount of improvement was greater at 8th week than that of the last visit (MD: 2.74; P = 0.04 and MD: 1.26; P = 0.06, respectively). According to Table 2, there was a significant superiority in favor of B. Carteri group at the first follow-up (P = 0.04) and a borderline significant difference was recorded at the last visit (P = 0.04). In a similar pattern, a significant improvement was observed in SSS section of Boston questionnaire in the weeks 0 - 8, and 0 - 12 in the two groups. Interestingly, the improvement in B. Carteri group was dramatically superior to that of the placebo group at both follow-up time-points (MD: 3.8; P = 0.001 for the 8th week and MD: 2.4; P = 0.01 for the 12th week).
The secondary clinical outcomes were further examined using electrodiagnostic parameters and assessment of pain free-grip strength (PFGS). Among them, only the grip strength significantly improved in both groups at weeks 8th and 12th compared to the baseline level. However, the statistical analysis showed no significant preference between the two groups (MD: 0.67; P = 0.3 and MD: 0.56; P = 0.4, respectively). On the other hand, according to the results of electrodiagnostic testing, SNAP and CMAP latency were almost similar between the groups and did not significantly change throughout the study (Table 2). The same results were also obtained regarding SNAP and CMAP amplitude, without any significant changes within and between the groups.
In the current study, triple-blinded method was used, therefore, due to allocation concealment, the selection bias was not noticeable. Also, baseline characteristics of the two groups were not significantly different. It was tried to have the least performance bias with blinding of participants and personnel. By blinding of outcome assessment, detection bias was somehow eliminated. Results of all patients that completed the study were reported; thus, there was no reporting bias.
5. Discussion
In the current study, the topical application of B. Carteri oleogel caused a significant improvement in VAS, SSS, FSS, and PFGS compared to the baseline level. putting grip-strength aside, this improvement was significantly superior to that of the placebo. Both VAS and SSS revealed that the topical oleogel of B. Carteri relieved symptoms more than placebo. However, there was no remarkable preference between the two groups regarding improvement of EDX parameters. To summarize, results of the current investigation showed a significant superiority for almost all clinical outcome measures in the intervention group (B. Carteri) rather than placebo group within three months follow-up. In the parallel group, placebo effect was detected; but as expected, the effectiveness did not remain until the end. Both VAS and SSS proved that the topical oleogel of B. Carteri achieved better symptom relief than placebo. In a similar manner, functional status of participants improved and this improvement was significantly greater in the experiment group After eight weeks of follow-up. Grip strength also changed, but the difference was not significant between the two groups. Meanwhile, the experimental oleogel could not change electrodiagnostic parameters in the current study. Therefore, no remarkable preference was observed between the two groups in terms of improvement in their grip strength or EDX parameters. In addition, to monitor any possible side effect of medication, adverse drug reaction reporting form was given to all patients. No complications were reported in the intervention group.
Based on the existing literature, various therapeutic effects including bactericidal, analgesic, and anti-inflammatory properties are attributed to B. Carteri. Hartmann et al. (34) showed that Boswellia extract could reduce edema in ulcerative colitis both directly, through antioxidant mechanism, as well as indirectly via increasing the antioxidant capacity of tissue throughout potentiation of some mediators including superoxide dismutase and glutathione peroxidase. Accordingly, Moussaieff et al. (42), demonstrated that phenolic compounds of Boswellia could protect neurons by reducing the inflammatory mediators. As described before, more recent laboratory and clinical investigations revealed that an extract derived from BC could be effective for some inflammatory conditions such as rheumatoid arthritis and osteoarthritis (OA) (24, 27, 29-32). Regarding the latter disease, authors’ previous study on 154 patients with knee OA in three groups showed that B. Carteri oleogel could significantly reduce osteoarthritic pain after six weeks usage, better than sesame oleogel (as placebo intervention) and the same as diclofenac gel (as standard treatment). Moreover, another study proved that the therapeutic efficacy of B. Carteri in symptoms relief and functional status improvement in Patients with OA was observed as soon as one week after starting topical treatment. Subjects began to show dramatic changes within one week of treatment with up to a 65% reduction in their pain scores, a Finding similar to the rapid onset of efficacy in the current trial (24).
Another double-blind RCT studied Boswellia in combination with Elaeagnus angustifolia, another herb with anti- inflammatory effects, in comparison to ibuprofen among patients with knee OA. Eventually, no superiority was observed between the groups. The mentioned herbal combination could efficiently decline pain and improve functional status among patients with knee OA. Therefore, authors concluded that Boswellia in combination with E. angustifolia was as effective as NSAIDs (ibuprofen) with a lower risk of gastrointestinal complications (22). These encouraging findings prompted the authors to investigate the efficacy of topical product of B. Carteri on symptom relief and functional improvement of patients with mild or moderate CTS in a triple-blind controlled trial. To the authors’ best knowledge, there is no similar previous study to compare the current study results with. A double-blind RCT in 2017 with a relatively similar design studied the efficacy of Chamomile (Matricaria chamomilla L.) oil, as another analgesic herb, among patients with mild or moderate CTS. They included 86 subjects with a confirmed diagnosis of non-severe CTS. Participants received either of the topical chamomile oil or placebo for a one-month period and were reassessed at the end of the study. Authors lastly found a significant symptom relief and improvement in functional scale in Chamomile group compared to placebo. Whereas, except for CMAP latency, the other electrodiagnostic variables revealed no dramatic difference between the two groups. Therefore, researchers did not observe any dramatic change in terms of electrodiagnostic parameters (22). The latter result was also obtained in the current RCT; the electrodiagnostic parameters did not change at all across three months of follow-up.
Similar to the prior successful results on DM, and knee OA, the current study findings revealed a significant effectiveness in clinical outcomes larger than placebo. In other words, a dramatic superiority was observed in VAS and two parts of Boston questionnaire, in favor of experimental group. On the other hand, a disappointing pattern was obtained using electrophysiological testing and PFGS measurements. Indeed, the present findings were in accordance to the prior experiments on the effectiveness of B. Carteri regarding symptom relief and functional status. It is speculated that the extract of BC might have no significant efficacy on the electrodiagnostic CTS parameters or grip-strength. It might be due to the small sample size or having not enough power to detect small differences. Also, it is possible that the follow-up period was not enough to meet the improvement in electrodiagnostic features.
The sample size was small, which is a limitation of the current study. However, this may be regarded as a preliminary study that showed promising results and it is recommended that studies with longer follow-up periods and larger population of patients with CTS be performed. Comparison of this oleogel with other interventions such as Carpal tunnel steroid injections is of value. Studying the efficacy of B. Carteri oleogel in patients with CTS and underlying diseases may be considered, too. Future investigations into the subject would certainly shed light on definite efficacy of this herbal oleogel.
5.1. Conclusions
In summary, it can be concluded that B. Carteri oleogel could improve pain and functional status better than placebo among patients with CTS. However, no electrodiagnostic change was detected in this period, probably due to the short-term follow-up.