1. Background
Coronavirus disease 2019 (COVID-19), which was firstly reported in Wuhan, China, in December 2019, became widespread in other countries. Accordingly, it was declared by the World Health Organization (WHO) as a pandemic on March 11, 2020. In Iran, the first case of the disease was reported in Qom on February 19, 2020, and afterward, this disease gradually spread to the other parts of the country. According to the experiences of China and some other countries, the disease is mild and is rarely associated with mortality in children. In this regard, the key diagnostic point in this age group is epidemiological exposure (1). Coronaviruses are isolated from four to six percent of hospitalized children due to respiratory infections. Unlike other respiratory viruses like respiratory syncytial viruses, the relative prevalence of coronavirus infections does not decrease along with age. Also, the importance of children in spreading the disease is still unclear. Adult data showed that shortness of breath, acute respiratory distress syndrome (ARDS), and death can also occur in children (2, 3).
Based on the recent reports, children show more gastrointestinal symptoms such as diarrhea, vomiting, and abdominal pain compared to adults (4). Moreover, unlike adults who are mostly affected by hospital contact, children are more likely to be involved in home contact (5-8). Although the clinical symptoms are mild, they can be known as a source of infection for adults (6). Also, mild symptoms in children may make physicians ignore the infection, which results in further disease spread.
2. Objectives
Given the fact that the disease is still novel and information on its different aspects in children is little, this paper aimed to report the clinical, paraclinical, and epidemiological features of the hospitalized children affected by COVID-19 in the southeast of Iran.
3. Methods
Data obtained from all children under the age of 15 years old who were hospitalized with the symptoms of acute respiratory infection were gathered from six hospitals in Kerman Province, Iran, from February 20 to May 14, 2020. The hospitals included two teaching hospitals in Kerman City and four general hospitals in other cities of Kerman Province. Accordingly, respiratory tract infection is defined as fever, cough, sore throat, and/or respiratory distress (9). Respiratory distress referred to summate a conglomeration of clinical features, including tachypnea, the use of accessory muscles of respiratory, lower chest wall indrawing, grunting, hypoxemia, and cyanosis (10). Besides, in this study, confirming the case for COVID-19 was performed in terms of the WHO guideline (11). The patients with positive nasopharyngeal PCR tests for the coronavirus or those who had radiological criteria were considered infected with COVID-19.
For each patient, demographic data such as age, sex, and past medical history data such as underlying diseases, antibiotics usage before admission, a history of routine vaccination, and influenza vaccination were collected. Also, data were collected on the symptoms of respiratory infection, including fever, cough, respiratory distress, and gastrointestinal symptoms. Subsequently, a nasopharyngeal specimen was collected from each patient by a trained health care worker. In this regard, nasopharynx sampling was performed using a Dacron swab through the nose and throat by rotating the swab in the nasopharyngeal area. Then the samples were sent to the university central laboratory for conducting real-time polymerase chain reaction (RT-PCR) test using special transport environments. Correspondingly, RT-PCR tests were performed using the kit approved by the WHO to diagnose the novel coronavirus. Also, other laboratory data, including blood cell count (WBC), lymphocyte and thrombocyte count, creatine phosphokinase (CPK) level, and lactate dehydrogenase (LDH) level, were collected.
Findings related to chest images, including computed tomography scans and X-rays of patients, were reported by an experienced radiologist. Interpretation and classification of the patient’s radiological findings were also performed using the guideline of the radiology association. compatibility was based on the results of the CT scan of the chest, according to the recommendations of the Iranian consensus expert statement (12).
We obtained informed consent from the children’s parents. Also, the study proposal was approved by the Ethics Committee of Kerman University of Medical Sciences on behalf of the National Ethics Committee of Iranian Biomedical Research (ethics code: IR.KMU.REC.1399.009).
A trained physician or a member of the study team collected the patient’s data using a data collection form. The data were then analyzed using SPSS version 20. The results were presented using descriptive statistics as mean ± standard deviation (SD) for continuous variables, and the number and percentage for categorical variables. Moreover, chi-square and Fisher’s exact tests were employed to compare the frequencies in different subgroups. A P value of less than 0.05 was considered statistically significant.
4. Results
A total of 97 children under the age of 15 years old with acute respiratory symptoms were hospitalized in Kerman Province hospitals. Near 57% (n = 55) of the patients were admitted to the general hospitals, and rest of them were hospitalized in the teaching hospitals (n = 42, 43.3%). The mean (SD) and the median age of the patients were 71.53 (62.0) and 57 months, respectively. Notably, forty-seven percent (n = 48) of the patients were female, and 52 % (n = 50) of them were male subjects. Ninety-four percent (n = 91) of the patients had no similarities or suspected symptoms in their family history. Also, twenty-seven percent (n = 26) of the children had an underlying disease. The most common underlying diseases were pulmonary disorders (9.2%) and immunodeficiency (3%). The routine vaccination was completed on 96% (93) of the participants but only 10% (n = 10) of the subjects received the flu vaccine in 2019. Overall, 32% (n = 31) of them had taken oral antibiotics before hospitalization.
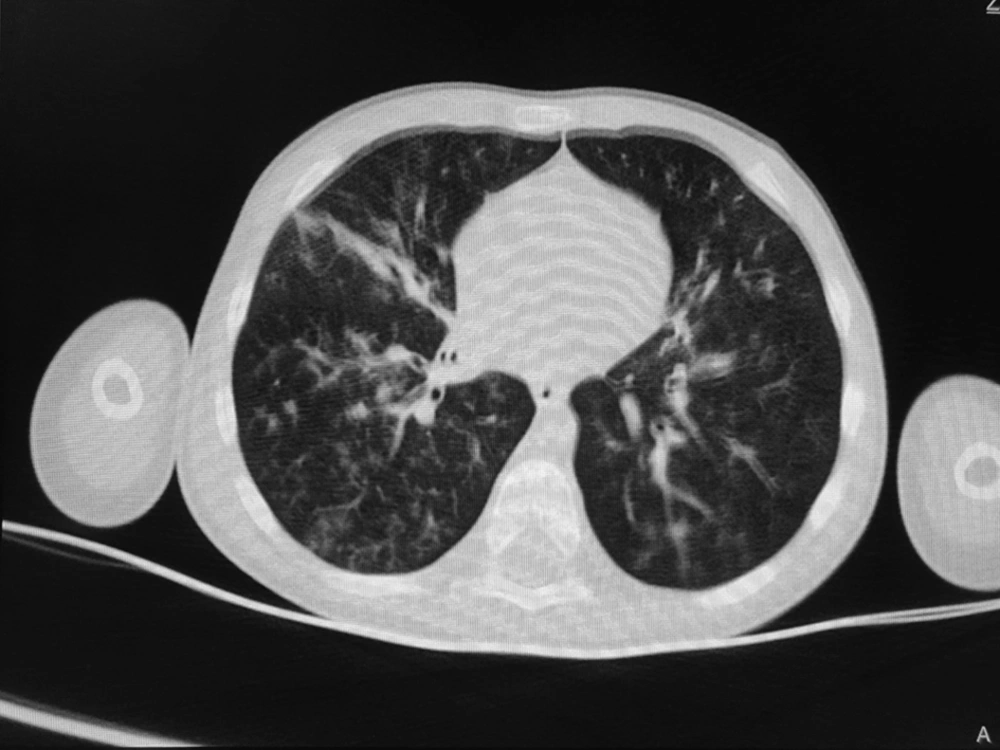
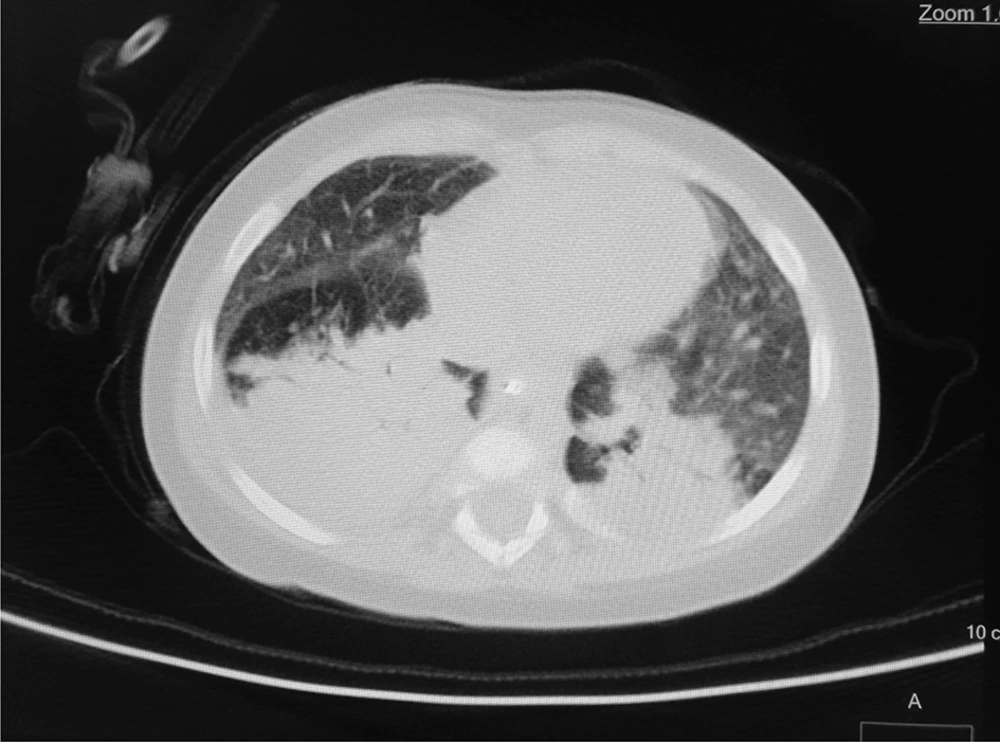
According to the WHO’s diagnostic criteria, 13.4% of the patients (n = 13) had been diagnosed by COVID-19. Six cases (6.2%) had positive RT-PCR results, and 9 cases (9.3%) were diagnosed COVID-19 according to their lung radiological findings (two patients with positive RT-PCR had radiologic involvements). Of nine patients with radiological criteria for COVID-19, eight patients showed bilateral patchy infiltration, and one patient showed the grand glass pattern on lung CT scan. In the patients with positive RT-PCR, two cases had bilateral patchy infiltration on the CT scan. The remaining RT-PCR-positive patients had normal lung CT scans (Figures 1 and 2).
The mean (SD) and median of age of the patients with COVID-19 were 68.0 (55.9) and 60 months, respectively. Six patients with COVID-19 were under 5 years old, and 9 ones (69.2%) were boys. Moreover, 11 (84.6%), 8 (61.5), and 5 (38.5%) patients with COVID-19 had a fever, cough, and respiratory distress, respectively. Also, 5 (38.5%) patients had gastrointestinal symptoms, including diarrhea, vomiting, or abdominal pain.
The frequency of antibiotic usage before hospitalization was significantly higher in non-COVID-19 subjects (69.2% vs. 26.2%) compared to the patients with COVID-19 (P = 0.004). Notably, there was no significant difference between the two groups in terms of gender, age, underlying diseases, routine vaccination, and influenza vaccination (P > 0.05) (Table 1).
| Variables | Categories | Total (N = 97) | COVID-19 (N = 13) | Non-COVID-19 (N = 84) | P Value |
|---|---|---|---|---|---|
| Sex | Female | 45 (46.4) | 4 (30.8) | 41 (48.8) | 0.255 |
| Male | 51 (53.6) | 9 (69.2) | 43 (51.2) | ||
| Age group, y | ≤ 5 | 51 (52.6) | 7 (53.8) | 44 (52.4) | 1.000 |
| > 5 | 46 (47.4) | 6 (46.2) | 40 (47.6) | ||
| Underlying diseasesb | Yes | 26 (26.8) | 6 (46.2) | 20 (23.8) | 0.103 |
| No | 71 (73.2) | 7 (53.8) | 64 (76.2) | ||
| Antibiotics use before admission | Yes | 31 (32.0) | 9 (69.2) | 22 (26.2) | 0.004 |
| No | 66 (68.0) | 4 (30.8) | 62 (73.8) | ||
| Routine vaccination | Complete | 93 (95.9) | 13 (100.0) | 80 (95.2) | 0.422 |
| Incomplete | 4 (4.1) | 0 (0.0) | 4 (4.8) | ||
| Influenza vaccination | Yes | 10 (10.3) | 1 (7.7) | 9 (10.7) | 1.000 |
| No | 87 (89.7) | 12 (92.3) | 75 (89.3) | ||
| Fever | Yes | 51 (52.6) | 11 (84.6) | 40 (47.6) | 0.016 |
| No | 46 (47.4) | 2 (15.4) | 44 (52.4) | ||
| Cough | Yes | 40 (41.2) | 8 (61.5) | 32 (38.1) | 0.136 |
| No | 57 (58.8) | 5 (38.5) | 52 (61.9) | ||
| Respiratory distress | Yes | 16 (16.5) | 5 (38.5) | 11 (13.1) | 0.022 |
| No | 81 (83.5) | 8 (61.5) | 73 (86.9) | ||
| Gastrointestinal symptoms | Yes | 23 (23.7) | 5 (38.5) | 18 (21.4) | 0.179 |
| No | 74 (76.3) | 8 (61.5) | 66 (78.6) |
aValues are expressed as No. (%).
bChronic lung diseases, immune deficiency disorder, developmental disorder, seizure.
Fever and cough with frequencies of 52.6% and 41.2% were the most common complaints of all the patients, respectively. The frequency of fever was significantly higher in the patients with COVID-19 compared to the non-COVID-19 patients (84.6% vs. 47. 6%, P = 0.016); however, there was no significant difference in terms of cough among them. More than 16% of all the patients had respiratory distress that the frequency of respiratory distress was higher in children with COVID-19 (38.5%, 5 of 13) than patients with non-COVID-19 (13.1%, 11 of 84) significantly (P = 0.022). Approximately 24% of the patients had at least one of the gastrointestinal symptoms, and the frequency of these symptoms indicated no significant difference between the two groups (P = 0.179) (Table 1). Vomiting, abdominal pain, and diarrhea with frequencies of 17.5%, 10.3%, and 6.2% were found to be the most common gastrointestinal symptoms, respectively.
Leukopenia, lymphopenia, and thrombocytopenia were present in 19.6%, 33.0%, and 20.7% of all the patients, respectively. Moreover, there was no significant difference between the groups in terms of the above-mentioned variables (P > 0.05). Furthermore, 28.1% (n = 27) and 13.7% (n = 13) of all the patients showed the abnormal level of LDH and CPK, respectively. Also, the frequencies of abnormal LDH (P = 0.735) and CPK (P = 0.160) showed no significant differences between the two groups (Table 2).
| Variables | Categories | Total (N = 97) | COVID-19 (N = 13) | Non-COVID-19 (N = 84) | P Value |
|---|---|---|---|---|---|
| Leukopenia | Yes | 19 (19.6) | 4 (30.8) | 15 (17.9) | 0.276 |
| No | 78 (80.4) | 9 (69.2) | 69 (82.1) | ||
| Lymphopenia | Yes | 32 (33.0) | 6 (46.2) | 26 (31.0) | 0.278 |
| No | 65 (67.0) | 7 (53.8) | 58 (69.0) | ||
| Thrombocytopenia | Yes | 20 (20.7) | 3 (25.0) | 17 (20.2) | 0.704 |
| No | 76 (79.3) | 9 (75.0) | 67 (79.8) | ||
| LDH | Yes | 27 (28.1) | 4 (33.3) | 23 (27.4) | 0.735 |
| No | 69 (71.9) | 8 66.7) | 61 (72.6) | ||
| CPK | Yes | 13 (13.7) | 0 (0.0) | 13 (15.5) | 0.160 |
| No | 82 (86.3) | 11 (100.0) | 71 (84.5) | ||
| ICU admission | Yes | 28 (28.9) | 5(38.5) | 23 (27.4) | 0.412 |
| No | 69 (71.8) | 8 (61.5) | 61 (72.6) | ||
| Antibiotic use during admission | Yes | 54 (55.7) | 7 (53.8) | 47 (56.0) | 0.887 |
| No | 43 (44.3) | 6 (46.2) | 37 (44.0) | ||
| Treatment outcome | Recovery | 89 (91.8) | 11 (84.6) | 78 (92.9) | 0.291 |
| Death | 8 (8.2) | 2 (15.4) | 6 (7.1) | ||
| Hospitalization duration |
aValues are expressed as No. (%).
Overall 56% (n = 54) of the patients received antibiotics during their time of hospitalization, and there was no difference between the patients with COVID-19 and non-COVID-19 patients (P > 0.05) (Table 1). Also, four patients (31%) with COVID-19 received Chloroquine as an antiviral drug.
The mean (SD) of hospitalization duration was 6.05 (4.96), 6.18 (4.79), and 6.02 (5.04) days in all patients, the children with COVID-19, and the non-COVID-19 subjects, respectively. There was no significant difference between the two groups of patients in terms of hospitalization duration (P = 0.815). During the hospitalization period, 28.9% of the patients were admitted in ICU who was 38.5% (n = 5) from the patients with COVID -19 and 27.4% (n = 23) from the non-COVID-19 patients (P = 0.668). There were two deaths among COVID-19 patients. An 8-month infant had a developmental disorder and seizure, and another one was identified with chronic lung disease. Overall, the death rate during the hospitalization period was 8.2% among all the patients that showed no significant difference between the patients with COVID-19 (15.4%) and the non-COVID-19 patients (7.1%) (P = 0.291) (Table 2).
5. Discussion
This study aimed to investigate the prevalence of COVID-19 in children who were admitted with acute respiratory symptoms in Kerman Province hospitals, southeastern Iran, during the 2020 epidemic. In this regard, 97 children were hospitalized with acute respiratory syndrome from February 20 to May 14, 2020. Out of them, thirteen patients were diagnosed as COVID-19 according to the criteria of the World Health Organization. During the same period, 817 adults were admitted to the hospitals in this area who were diagnosed to be infected by COVID-19. Severe involvement and hospitalization have also been lesser in children in other studies compared to adults (13). In a large study performed in China, only 2.1 percent of the patients were under 19 years old (14). Findings from other studies also showed that children are less involved than adults and usually recover within 7 days (15).
There are several reasons why children are less likely to have severe illness compared to adults. Explaining, children’s T cells’ immune system works differently against childhood pathogenic viruses, which has a cross-immunity with different viruses. Besides, various vaccinations that are given in childhood increase the strength of immunity. The immune system of children is evolving and still has not become as active as adults for self-attacking. Moreover, virus receptors, like ACE, are different in children and adults (16).
On the other hand, a low incidence of the disease in children could be due to mild symptoms and lack of attention and identification of the patients. Also, false-negative diagnostic molecular tests in children, closure of kindergartens, and schools during an epidemic are known as the other causes of the low incidence of the disease in children (15).
Out of 97 patients who were hospitalized due to acute respiratory syndrome, 26 (27%) had an underlying disease. Correspondingly, there was no significant difference between the two groups in this regard. The epidemic of COVID-19 in the region began at the end of the acute respiratory illnesses season, resulting in a decrease in the acute respiratory viral illness and a lower number of hospitalized patients. Because of the fear of infecting by a coronavirus, many patients with mild respiratory problems, who had no underlying disease, preferred to not be admitted to a hospital.
The history of antibiotic usage before hospitalization was significantly lower in children who were ultimately diagnosed with COVID-19 compared to the other patients. In this regard, it could be potentially due to the low incidence of bacterial infections such as otitis media and sinusitis following COVID-19 (17). Another cause of the lower rate of antibiotic use in children with COVID-19 may be due to a more rapid progression of respiratory diseases than the non-COVID-19 group.
Fever and cough were known as the most common clinical symptoms. Fever was significantly higher in patients with COVID-19 than in non-COVID-19 patients, and this finding is consistent with other studies (14). Also, other symptoms, such as gastrointestinal symptoms, were not different between the COVID-19 and non-COVID-19 patients. No study compared the COVID-19 and non-COVID-19 patients concerning their gastrointestinal symptoms, but in some reports, children showed more gastrointestinal symptoms than adults (4).
There was no significant difference between the COVID-19 and non-COVID-19 patients in terms of laboratory findings such as WBC, leukopenia, lymphopenia, thrombocytopenia, LDH level, and CPK level. The rate of leukopenia was observed more frequently in the COVID-19 patients than non-COVID-19 ones. Although this difference was not statistically significant, it might be due to the low number of patients in the COVID-19 group. The same pattern was observed in regard to lymphopenia in our patients. Notably, in a study, lymphopenia had been relatively common, and a high CRP level has been cited as a marker of the disease severity in adult patients with COVID-19 (18, 19).
In this study, the most common findings in chest CT scans of the patients with COVID-19 were bilateral patchy infiltration, and only one patient had a view of the ground glass opacity. In a similar study performed in the north of Iran, grand glass appearance has been reported in 25% of patients (8). Other studies in other countries have also shown ground glass involvement in a small percentage of children with COVID-19 (20).
Four (31%) patients with COVID-19 and 23 cases (27%) out of non-COVID-19 patients needed special care in the intensive care unit. There was no significant difference between the two groups in terms of intensive care, antibiotic usage, and the need for oxygen, which is consistent with the results of similar studies (18). Also, four patients (31%) diagnosed with COVID-19 had received chloroquine as an antiviral drug. Also, in other similar studies, a small percentage of the patients received antiviral drugs (21).
In this study, the overall mortality rate was 8%. However, this mortality rate appears to be high, but due to the epidemic of COVID-19 and the fear of hospitalization, most of the patients were ill with an underlying disease. Out of 13 COVID-19 patients, there were two deaths. An 8-month infant had a developmental disorder and seizure, and also, another one had chronic lung disease. Although mortality is low in children with the disease, it can occur in patients with underlying diseases (22). The most important limitation of this study was the low number of the included patients. For a more detailed comparison, a larger study should be proposed at the national level.
5.1. Conclusions
Compared to the adults, the incidence of COVID-19 is lower in children hospitalized with acute respiratory syndrome. Severe complications of the disease and mortality occur in those children with underlying diseases. Most of the children with COVID-19 recover with supportive care with no need for specific treatment.