1. Background
Liver transplantation is a proven therapeutic modality for end-stage liver diseases with distinct etiologies. Despite the significant improvements in patients’ conditions, problems that frequently happen following transplantation can have adverse effects on their lives (1). One of the complications of liver transplantation is post-Transplant lymphoproliferative disorder (PTLD), which affects patient survival and graft function. Moreover, children who undergo organ transplantation are at risk of developing lymphoproliferative disorders, with Non-Hodgkin lymphoma (NHL) being the most serious disease (2) whose definitive diagnosis requires histopathological examination. In these patients, PTLD is commonly considered a result of immunodeficiency caused by the consumption of immunosuppressive medications, eventually leading to depressed T cell activity and lymphoid proliferation (3, 4). Also, PTLD is usually an Epstein-Barr virus (EBV)-associated condition; approximately 60 - 70% of the patients are EBV-positive, with the lack of a cytotoxic T cell response due to the suppression of the immune system (5-8).
Advances in biology as well as the promotion of knowledge about the epidemiology and diverse clinical presentations of PTLD have led to the identification of patients at risk of PTLD after solid organ transplantation (9). Young age is one of the known risk factors that lead to PTLD. In fact, this disorder is more common amongst pediatric patients (10). Support for infants is partly provided by mothers’ immune system, while their endogenous responses to puberty continue until childhood and adolescence. This period is accompanied by most physical and hormonal changes, except for pregnancy, causing differences in the risk of PTLD and the related risk factors in different pediatric age groups (11-14). Furthermore, PTLD is more common in recipients undergoing long-term treatment with immunosuppressive drugs after transplantation. These treatments are varied, but they basically include immunosuppression reduction, anti-CD20 antibody, surgery, radiotherapy, and chemotherapy (15). The use of effective treatment methods such as rituximab (RTX) and Sirolimus, as well as decreasing the dosage of immunosuppressive drugs, has resulted in a significant improvement in health conditions and the overall survival (16, 17). For instance, preventive therapy using RTX has been found to lead to a temporary decrease in the EBV level (18).
2. Objectives
Based on what was mentioned above, the present study aimed to investigate the demographic and clinical features of PTLD among Iranian patients after liver transplantation.
3. Methods
Shiraz Transplant Hospital (Shiraz, Iran) is Iran’s main center, with significant pediatric liver transplant cases annually. This cross-sectional study was conducted on the patients aged < 18 years who had undergone liver transplantation at this center from April 2007 to March 2017. The inclusion criteria of the study were aging < 18 years and having undergone liver transplantation. The exclusion criteria were incomplete demographic information in hospital files and death due to transplant complications in the first week after transplantation.
The donors’ and recipients’ epidemiological and clinical features were obtained using a form including information about age, gender, underlying liver disease, type of graft (partial, split, and whole organ), age at liver transplantation, time of PTLD development, multi-organ involvement, immunosuppressive regimen (tacrolimus, sirolimus, cellcept), and dosage and period of consumption of immunosuppressive drugs prior to PTLD. Here, PTLD was diagnosed by histopathological biopsy specimens and was confirmed by pathologists according to the World Health Organization (WHO) classification (19). After that, screening work-ups such as computed tomography (chest, pelvis, and abdomen), bone marrow aspiration, and biopsy were done to assess the likelihood of multi-organ damage. The patients’ survival rate after PTLD was also recorded. The cumulative survival proportion was compared between males and females as well as between the patients aged below and above six years. The differences between different sex and age groups in terms of survival were evaluated via the log rank test. The data were analyzed using the SPSS software, version 22.
4. Results
Out of the 1207 pediatric patients who had undergone liver transplantation in Shiraz Organ Transplant Center, Shiraz, Iran, from April 2007 to March 2017, 49 were diagnosed with PTLD following liver transplantation, representing a prevalence of 4%. The mean age of the transplant recipients who developed PTLD at the time of transplantation was 4.93 ± 1.07 years, ranging from 11 months to 9 years. The mean age at transplantation was 7.80 ± 5.54 years in the non-PTLD cases. In addition, the female-to-male ratio was 51.49% in the PTLD group and 44.56% in the non-PTLD group. The recipients’ mean weight was 14.828 ± 0.96 kg at the time of PTLD. Biliary atresia was the most common underlying disease in both PTLD and non-PTLD patients, with 12 and 148 cases per 1,000 patients, respectively. The second most common underlying diseases were Crigler-Najjar disease in the patients with PTLD (9 cases per 1000 patients) and Wilson disease in the non-PTLD patients (168 cases per 1,000 patients). Of the 49 patients with PTLD, 28 (57%) had received a partial graft, while 11 (23%) had received a whole organ transplant (Table 1). Organ involvement was also assessed in the 49 patients with PTLD. The results indicated that the liver (8%), bowel (8%), and cervical (8%), and submandibular lymph nodes (8%) were the most affected sites (Table 2).
| Demographic Data | PTLD | Non-PTLD |
|---|---|---|
| Mean age at the time of liver transplantation (y) | 4.93 ± 1.07 | 7.80 ± 5.54 |
| Sex, female | 25 (51) | 508 (44) |
| Underlying disease | ||
| Autoimmune hepatitis | 1 (2) | 92 (7.9) |
| Biliary atresia | 15 (30.7) | 179 (15.5) |
| Budd-Chiari | 1 (2) | - |
| Crigler-Najjar | 11 (22.5) | 83 (7.2) |
| Hepatocellular carcinoma | 1 (2) | 11 (0.9) |
| Progressive familial intrahepatic cholestasis | 8 (16.4) | 152 (13.1) |
| Tyrosinemia | 8 (16.4) | 106 (9.1) |
| Tyrosinemia superimposed with hepatocellular carcinoma | 1 (2) | - |
| Wilson disease | 1 (2) | 168 (14.5) |
| Hyperoxalouria | - | 17 (1.5) |
| Acute liver failure | - | 32 (2.8) |
| Primary sclerosing cholangitis | - | 20 (1.7) |
| Hypercholesterolemia | - | 50 (4.3) |
| Neonatal hepatitis | - | 37 (3.2) |
| Cryptogenic cirrhosis | - | 113 (9.8) |
| Missing cases | 2 (4) | 98 (8.5) |
| Allograft | ||
| Living donor | 32 (65) | 470 (40) |
| Cadaver | 17 (35) | 688 (60) |
| The graft types in the recipients | ||
| Partial | 28 (57) | 463 (40) |
| Split | 10 (20) | 139 (12) |
| Whole organ | 11 (23) | 556 (48) |
aValues are expressed as mean ± SD or No. (%).
| Variables | Values |
|---|---|
| Mean immunosuppressive drug consumption (mg/kg) | |
| Tacrolimus | 0.27 ± 0.23 |
| Sirolimus | 0.10 ± 0.08 |
| Prednisolone | 0.67 ± 0.62 |
| Cellcept | 0.07 ± 0.12 |
| Organ involvement | |
| Liver | 4 (8) |
| Multi organ involvement | 1 (2) |
| Mass in abdomen | 4 (8) |
| Axillary | 1 (2) |
| Bowel | 4 (8) |
| Cervical lymph node | 4 (8) |
| Inguinal lymph node | 1 (2) |
| CNS | 2 (4) |
| Colon | 1 (2) |
| Duodenum | 2 (4) |
| Heart | 1 (2) |
| Kidney | 1 (2) |
| Para aortic mass | 1 (2) |
| Submandibular lymph node | 4 (8) |
aValues are expressed as mean ± SD or No. (%).
The results of the comparison of the two groups regarding the mean dose/kg of immunosuppressive drugs have been presented in Table 2. Accordingly, the highest dose was related to prednisolone with the mean value of 0.67 ± 0.62 mg/kg. Additionally, the immunosuppressive drugs were used for 14.79 ± 14.40 months from the time of transplantation until the date of PTLD diagnosis. The five-year survival rate was 31% in the patients diagnosed with PTLD compared to 72.7% in the non-PTLD patients.
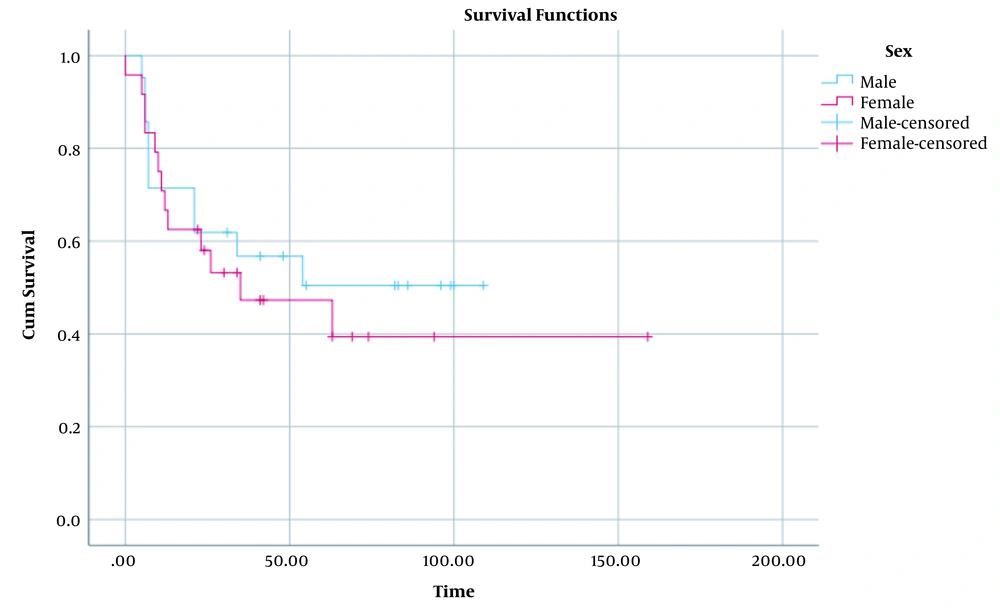
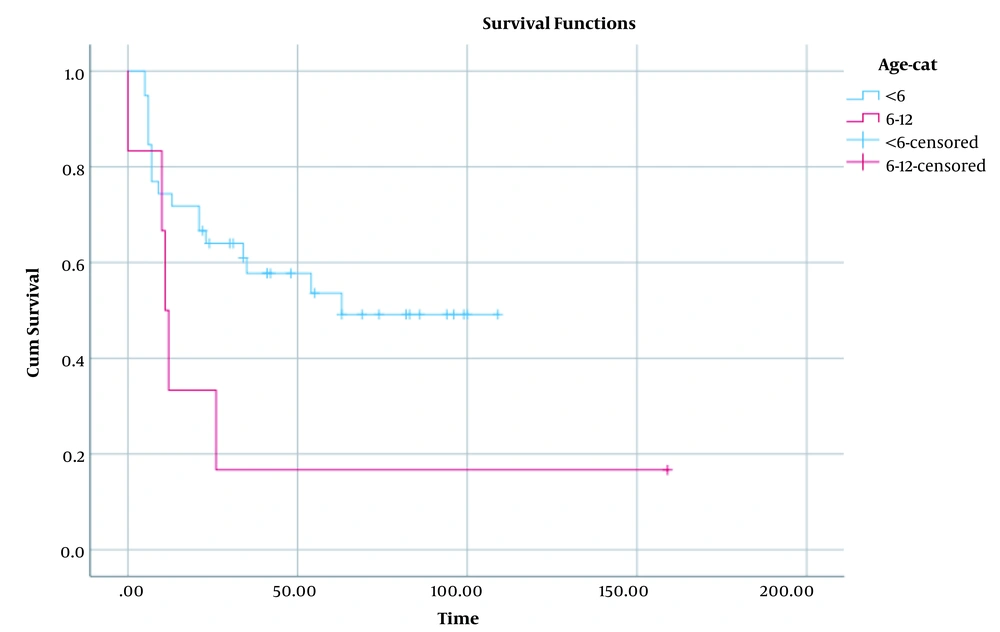
The epidemiological characteristics of the donors (n = 49) of the recipients who developed PTLD were evaluated in this study. Based on the results, the mean age of the donors was 25.28 ± 1.2 years (range = 3 - 45 years). Besides, most of them had a familial relationship with the recipients (Table 3). Comparison of the cumulative survival proportion in male and female patients with PTLD is depicted in Figure 1 (P = 0.59). Additionally, comparison of this proportion in < 6 and 6 - 12 age groups has been shown in Figure 2 (P = 0.06). Accordingly, the cumulative survival proportion was higher in males as well as in the patients aged under six years compared to females and the patients over six years old. The significance of these differences was evaluated using a log rank test. The results revealed no significant difference in the distribution of the survival proportion on the basis of these two factors (sex and age groups) (P = 0.59 and P = 0.06, respectively).
| Donors’ Characteristics | PTLD | Non-PTLD |
|---|---|---|
| Mean age (y) | 25.28 ± 1.2 (3 - 45) | 21.71 ± 13.40 (1 - 83) |
| Relationship with the recipient | ||
| First-degree relative | 31 (63.4) | 444 (38.4) |
| Second-degree relative | 1 (2) | 26 (2.2) |
| Cadaver | 17 (34.6) | 688 (59.4) |
aValues are expressed as mean ± SD or No. (%).
5. Discussion
This study was conducted on the epidemiological characteristics of the pediatric graft recipients with PTLD and their donors following liver transplantation. The results demonstrated that the mean age at transplantation was lower in the patients with PTLD than in the non-PTLD children (4.93 vs. 7.80 years). Haung et al. (20) reported a mean age of 4.1 years at the time of transplantation. In another study by Barış et al., the mean age at transplantation was 2.71 ± 3.21 years, and low age at transplantation, especially ages < 2.5 years, was considered a risk factor for the occurrence of PTLD (21). These results were consistent with those of the present investigation.
In the current study, the most common underlying disease was biliary atresia (30.7%). High rate of biliary atresia in patients with PTLD has been reported in other studies, as well. For instance, Wiederkehr et al. disclosed that 57.1% of the patients were diagnosed with biliary atresia prior to PTLD, which accounted for the majority of the cases (22). In the study carried out by Haung et al., biliary atresia was also detected in 66.7% of the patients (20).
In the present research, 57% of the cases had received partial transplants, and 23% had received whole organ grafts. In addition, first-degree relatives (parents and siblings) comprised 63.4% of the donors. Borenstein et al. also performed a study on 13 patients in need of transplantation and indicated that all the 13 donors were selected from living individuals, 12 of whom were the first-degree relatives of the recipients. Additionally, all the patients received partial transplants, and only 23% showed PTLD complications (23). Lozano et al. revealed better results in whole organ transplant recipients in terms of transplant maintenance and post-transplant complications (24), which was in agreement with the current study results. In the present study, the mean age of the graft donors was 25.28 ± 1.2 years. According to Tiao et al., donor’s age < 6 months or > 50 years was found to be a risk factor for a decrease in the lifespan of the recipients and transplants (25).
The current study findings demonstrated that the abdomen, liver, cervical lymph nodes, and submandibular lymph nodes were the most common locations affected by PTLD. A previous study by Barış et al. also revealed the liver, peripheral lymph nodes, and gastrointestinal system as the most common sites of involvement (21).
In the present research, the mean dose of tacrolimus consumption was 0.27 mg/kg from the date of transplantation until the occurrence of PTLD. The mean consumption doses of prednisolone, cellcept, and sirolimus were also 0.67, 0.07, and 0.10 mg/kg, respectively. However, Jeong et al. conducted a study on 20 patients over 20 years of age and measured the mean dose of tacrolimus as 0.15 - 0.22 mg/kg. Moreover, the duration of drug use from the time of transplantation until PTLD occurrence was 14.79 ± 0.96 months in the present study. This measure was found to be 15.63 months in another research (26), which was close to the current study results. It should be noted that the two groups under the current investigation could not be compared in terms of the cumulative immunosuppressive drug dose, weight, and age at transplantation (missing data).
In the current study, the survival rate of the patients was an average of 63% after transplantation. The findings of a similar study conducted in Shiraz between 2004 and 2015 revealed a six-month survival of 75.1 ± 6%, a one-year survival of 68.9 ± 6.5%, and five-year survival of 39.2 ± 14.2% after transplantation (10). In research conducted on 54 patients with PTLD at Florida University from 1994 to 2017, the mean follow-up was 28.8 months, and the average five-year survival rate was 87.6% for all age groups (95% CI: 74.3 - 94.2) (27). The differences were also assessed between different sex and age groups regarding the survival rate in the present research. The results revealed no significant difference between different sex and age groups concerning the survival distribution (P = 0.59 and P = 0.06, respectively).
Liver transplant recipients acquire immunodeficiency due to drug consumption. Immunocompromised patients are prone to complications, particularly opportunistic infection and oncogenic virus-associated malignancy. EBV-associated PTLD is also one of the catastrophes that may happen in transplant recipients. In a study conducted by Weisert et al. on heart transplant recipients, EBV was considered a risk factor, but the frequency of EBV screening varied among patients (28). Seo et al. assessed patients under 18 years old who had received liver transplants by a detailed analysis of the EBV blood level from January 2006 to March 2015. In children, the prevalence of PTLD was 10% after transplantation. Besides, the results of the multivariate analysis indicated that primary cytomegalovirus (CMV) infections and high-level EBV DNAemia after transplantation were linked to a higher risk of PTLD. Increased EBV viral load with the cut-off value of 44,000 copies/mL/week was associated with an increased risk of PTLD with a sensitivity of 64.3% and a specificity of 70.9% (29). Another study carried out in China also suggested that close monitoring of EBV DNA loads and checking the tacrolimus concentration might be useful in preventing the occurrence of PTLD amongst children after liver transplantation (30). Similarly, Chen et al. recommended the tapering of immunosuppressants in case of high EBV viral load in children (31). The above-mentioned studies revealed the importance of routine screening of EBV and CMV infections. However, EBV screening and viral load monitoring were not routinely performed for the recipients in the present study, which was the major limitation of the research. Therefore, routine viral monitoring is recommended for better evaluation and treatment of pediatric patients with PTLD.
5.1. Conclusions
The prevalence of the clinical and epidemiological features of PTLD in the patients with liver transplants was similar to that of the patients in other hospitals. Monitoring of EBV viral load in transplant recipients can provide a basis for managing patients and increasing their life expectancy.