1. Context
Since December 2019, the COVID-19 outbreak has turned into a significant global public health threat (1, 2). According to studies, this disease is transmitted person-to-person via respiratory viral droplets (3, 4), which led to the COVID-19 pandemic. Pregnant women and fetuses are among the high-risk groups for COVID-19. Due to the rapid spreading of COVID-19, fetal health is a serious concern. Nonetheless, sufficient data are not available regarding COVID-19 evaluation and management in pregnant women, and the possible vertical transmission risk is unclear (5).
The placenta, an entirely specialized organ, is vital for normal fetus growth and development during pregnancy (6). It supplies the fetus with oxygen and nutrients and removes the wastes and carbon dioxide. Besides substance transfer, the placenta acts as a barrier and prevents fetal exposure to potentially dangerous substances in the maternal blood. However, it cannot completely exclude all dangerous substances and prevent fetal exposure. Moreover, the placenta, as well as metabolizing some substances. Nevertheless, the virus passage from the placental barrier depends on the placental membrane’s physical properties and the viral particle biological properties. We intended to assess whether SARS-CoV-2 could cross the placenta. For this purpose, SARS-CoV-2 entry, pathogenicity, and replication methods were discussed in addition to the placental defense methods. Substantial research and time are required to understand how this virus may involve the fetus.
2. The Life Cycle of SARS-CoV-2 and the Pathogen Characteristics
2.1. Morphology, Structure, and Stability of SARS-CoV-2
The large pleomorphic spherical coronavirus particles have surface crown-like projections. The coronavirus diameter ranges from 75 nm to 160 nm (an average of 120 nm) (7, 8). The virion of SARS-CoV-2 is spherical and has a mean diameter of 78 nm. A lipid bilayer covers this virus where the envelope (E), membrane (M), and spike (S) structural proteins are located. As a beta coronavirus subset, SARS-CoV-2 has shorter spike-like surface proteins, which are known as hemagglutinin esterase (HE) (9). A nucleocapsid is inside the envelope, which is formed by several copies of nucleocapsid (N) proteins, which are attached to the single-stranded positive-sense RNA genome in an uninterrupted beads-on-a-string type shape. The genome length ranges between 26 and 32 kilobases (kb), which is the largest genome among the RNA viruses. This genome codes two proteases (polyproteins) (10).
Usually, its molecular weight is 5.5 - 6.1 × 106 (11). The membrane proteins, lipid bilayer envelope, and nucleocapsid protect this virus out of the host cell. Coronaviruses are stable at 4°C, room temperature (20°C,) and 37°C for a minimum of 2 h without a significant change in their infectious ability. However, they are noninfectious when exposed to 56°C, 67°C, and 75°C for 90, 60, and 30 min, respectively. Sixty minutes of UV irradiation destroys the viral infectivity in the culture medium at an undetectable level. Evidence suggests the great survival of the coronavirus in the environment and human specimens. It should be noted that UV irradiation and heating are effective methods for the eradication of viral infectivity (12, 13).
2.2. Pathogenicity and Entry Methods of SARS-CoV-2
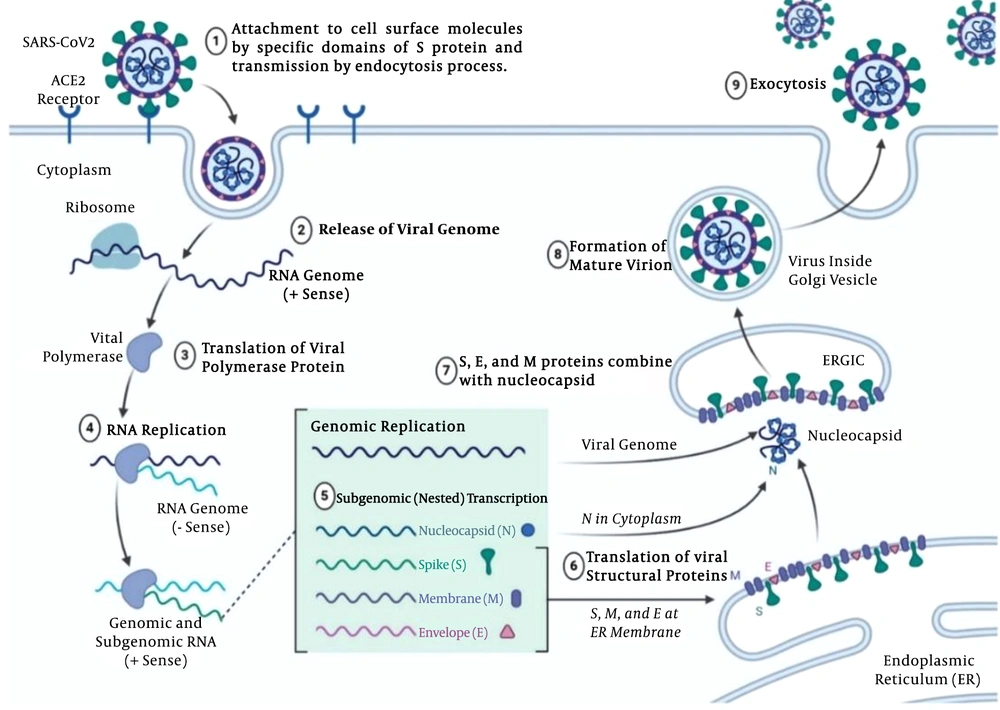
First, the virus should cross the cell membrane, which is rich in cholesterol and sphingolipid. The SARS-CoV-2 envelope should be bound to the membrane of the host cell for delivering the nucleocapsid to the host cell. Thanks to specific domains of protein S, SARS-CoV-2 binds to the surface molecules of the cell. The virus entry is mediated by the spike (S) glycoprotein, which is a key factor and known as the SARS-CoV-2 cell receptor, and plays a significant role in disease transmission and human infection (Figure 1) (14).
SARS-CoV-2 Life cycle and pathogenicity in nine stages; the virus-cell entry through the endocytosis process via ACE2 receptors; viral replication by polymerase and producing negative-strand full-length copies of the genome to provide mRNAs for the viral genes; viral particle gathering in the Golgi; viral particle release by exocytosis (figure was made using biorender.com).
Using receptor binding, proteolytic activation, and low pH exposure, the protein S shape is changed for cell entry (15). Cathepsin L, a protease associated with endosomal membranes, promotes viral infectivity through transforming the uncleaved S polypeptides to S1 and S2 proteins. Multiple strategies are available for spike protein proteolytic activation, such as cell surface transmembrane protease/serine (TMPRSS) proteases, trypsin, and furin. The host cell serine protease TMPRSS2 primes the SARS-CoV-2 S protein for cell entry (16, 17).
2.3. Replication
After cell entry, SARS-CoV-2 should replicate in the cytoplasm in which viral RNA is replicated (18). Replicase, a viral RNA polymerase, is the first gene that is translated, which produces negative-strand full-length copies of the genome to provide mRNAs for the viral genes. In an infected cell with SARS-CoV-2, large granular areas may be detected, which contain viral RNA and proteins. The viral particles are collected in the Golgi body and vesicles, where the virus particles are transferred to the surface of the cell to be released by exocytosis (Figure 1) (19).
3. Defense Methods of Placenta
3.1. Physicochemical and Structural Properties of Placenta Against SARS-CoV-2
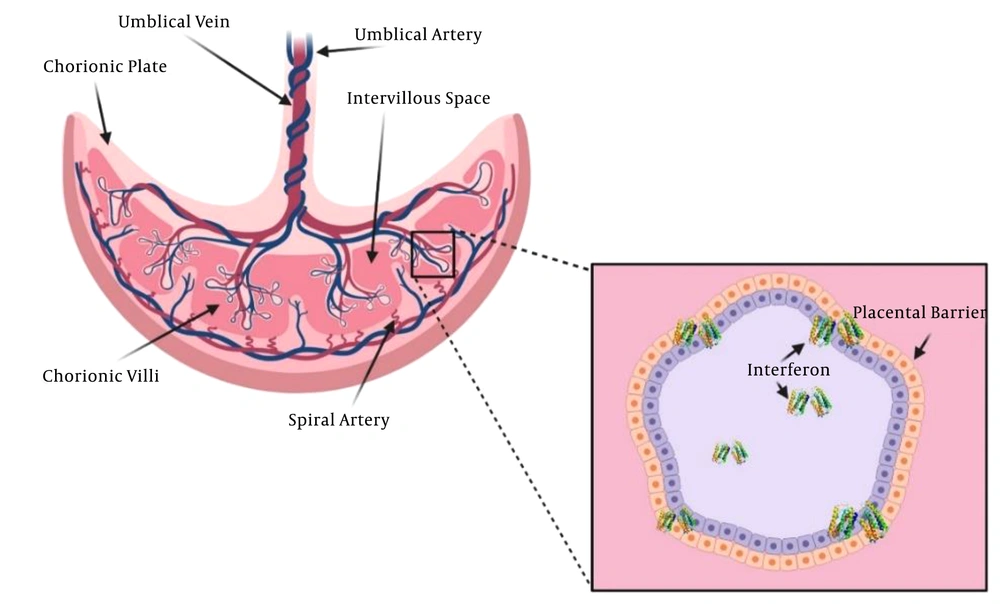
As a mediator between the fetus and mother, the placenta plays several vital roles. It prevents fetal allograft rejection, protects the fetus, exchanges the nutrients and respiratory gases, removes fetal waste products, and releases steroid and peptide hormones, which are crucial for fetus growth and development. The placenta syncytiotrophoblast layer is a major site for respiratory gas and nutrient exchange between the fetus and the maternal bloodstream (Figure 2) (20, 21). Several mechanisms are involved in this exchange: endocytosis, exocytosis, transcellular transfer (22), simple diffusion (23), and solvent drag (24). A receptor may mediate exocytosis and endocytosis processes, which is stimulated by a specific solute-receptor interaction on the cellular membrane (25). The transfer is affected by many factors, including the surface area, thickness, and metabolism of the planta, as well as fetal and maternal blood pH, uteroplacental blood flow, viral molecular weight, presence of viral transporters of the placenta, protein binding, lipid solubility, cross-placental concentration gradient, degree of ionization, and molecular weight (20, 26).
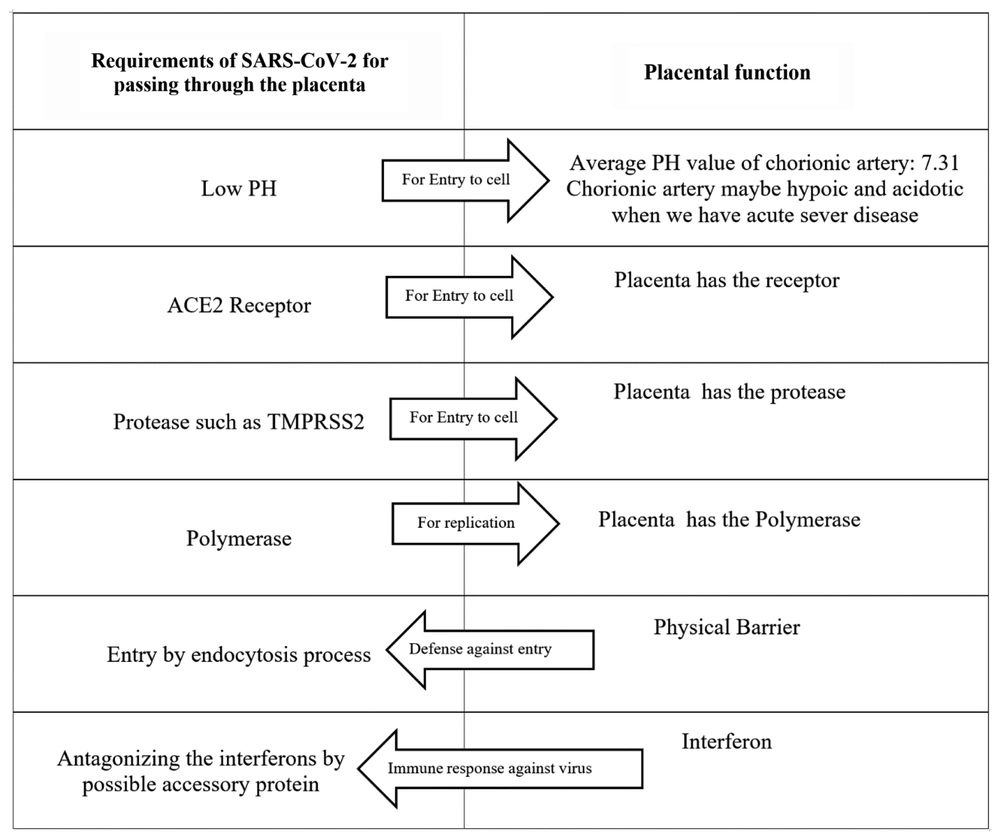
With a molecular weight of 5.5 - 6.1 × 106, SARS-CoV-2 may cross the placenta through the endocytosis process. The average chorionic artery pH is 7.31 (27). As known, SARS-CoV-2 requires a low pH for cell endosomal entry. The receptor of SARS-CoV-2, ACE2, is widespread in the cells of the maternal-fetal interface such as stromal cells, placental cytotrophoblast, and syncytiotrophoblast, as well as perivascular cells of the decidua. The high ACE2 expression in these cells shows that the placenta may be potentially infected with SARS-CoV-2 (28). Proteases, such as TMPRSS2, are required for the conformational change of protein S. Besides, TMPRSS2 mRNA expression is reported in the placenta (29).
The polymerase is required for SARS-CoV-2 replication. Three kinds of polymerases are present in the placenta. The activity of them is high in early pregnancy due to placental formation and mitosis, though it is low during the second trimester. The polymerase β activity does not change remarkably during pregnancy (30).
3.2. Hormonal and Immune Protection of the Placenta
de Goffau et al. (31) failed to find supporting evidence for the presence of the placental microbiome. However, they recognized a significant pathogen, S. agalactiae, in the placenta of nearly 5% of women before labor onset (31). The placenta is a tough immune organ by itself. When exposed to pathogens, it expresses pattern recognition receptors (PRR) and, in this way, responds to pathogens at the fetal-maternal interface (32, 33).
A study showed that SARS-CoV-2 pathogenesis is mainly caused by the inflammatory response (34). The innate immune system is the first line of defense against this pathogen. The most significant immunomodulators are the placental type interferons (IFNs), which support pregnancy. In the case of a viral infection, interferons I and III function as the antiviral defense responses (35, 36). The interferon I antiviral activity directly inhibits the replication of the virus. It also mediates both innate and adaptive cellular immune functions to produce resistance against viral infections and manage long-term immunity. Interferons I and III are strong placental antiviral proteins (Figure 2) (37-39).
Some viruses can inhibit the interferon I pathway and thus lead to the replication of the virus and infection. A great deal of the structural, nonstructural, and accessory proteins of coronaviruses have been shown to antagonize the response of IFNs. Besides the proteins, the genome of the coronavirus also encodes non-coding RNAs (ncRNAs). An N gene small ncRNA inhibition has been associated with reduced inflammation (40). These data are in line with the hypothesis stating that SARS-CoV-2 may potentially cross the placenta (Figure 3). Yet, can the crossed virus cause fetal infection? SARS-CoV-2 is a novel and an unknown virus. Many studies are required to further learn about SARS-CoV-2.
4. Evidence Supporting SARS-CoV-2 Crossing the Placenta
The Zeng et al. (41) study of antibodies in the infants of mothers suffering from COVID-19 pneumonia demonstrated elevated IgG levels in five infants and IgM detection in two infants at birth. Although IgG antibodies passively transfer across the placenta, IgM antibodies do not usually cross the placenta due to their larger molecular sizes. Hence, IgM might have been synthesized by the infant in the case of the SARS-CoV-2 cross (41). In another study, Algarroba et al. (42) identified coronavirus virions invading syncytiotrophoblasts in placental villi by using electron microscopy in a woman at 28 weeks’ gestation due to severe COVID-19 infection. Vivanti et al. (43) demonstrated the transplacental transmission of SARS-CoV-2 in a neonate born to a mother infected in the last trimester presenting with neurological compromise, confirmed by comprehensive virological and pathological investigations.
5. Evidence Against SARS-CoV-2 Crossing the Placenta
The only published study on the placental pathology of seven pregnant women with SARS-CoV-2 was conducted by Ng et al. (44), which reported abnormal placenta in the acute phase of COVID-19, as well as COVID-19 cases in the third trimester. The pathology reports indicated elevated intervillous and subchorionic fibrin and diffuse fetal thrombotic vasculopathy in the avascular chorionic villi areas. Interestingly, villitis, as an important manifestation of placental inflammation, was not recognized. Villitis is a major histologic sign in various maternal infections, which are hematogenously transmitted to the fetus via the placenta (44).
Schwartz (45) investigated 38 pregnant women who suffered from COVID-19. They reported that SARS-CoV-2 was not transmitted through the intrauterine or transplacental routes from infected pregnant women to the fetuses (45). They reported two neonatal COVID-19 cases, both of which failed to provide sufficient evidence to prove vertical transmission due to delayed testing or the potential transmission through a postpartum-infected contact (46, 47). Also, Chen et al. (48) did not report any evidence of positive COVID-19 diagnostic tests or symptoms in the infants of symptomatic, test-positive mothers. In another paper (letter to the editor), Kniss (49) proposed that the structures identified by Algarroba et al. are clathrin-coated vesicles, not SARS-CoV-2 particles (42).
6. Conclusions
A very serious complication of viral infections during pregnancy is vertical transmission. Except for the herpes virus, the maternal-fetal transmission of viral diseases commonly occurs via the hematogenous route. Teratogenicity and fetal growth and development impairment are among the known adverse effects of viral transplacental passage. This highlights the significance of investigating potential placental involvement in pregnant women who are infected with SARS- CoV-2.
Although the data is in line with the hypothesis of the SARS-COV-2 passage through the placenta, the placental defense system protects clinically against significant placental infection. It should be noted that intrauterine fetal growth restriction and maternal comorbidities, including gestational diabetes and preeclampsia, may damage the placental barrier by several mechanisms such as abnormal cytotrophoblast differentiation, defective placental angiogenesis, and placental ischemia. These mechanisms increase the risk of SARS-CoV-2 intrauterine transmission to the fetus. The growing knowledge of the placental defense mechanisms, as well as the immunological and hormonal responses, may help develop new antiviral agents.