1. Context
Learning and memory are the most important concepts in neuroscience. Learning can be defined as the mechanism by which new knowledge about the world is attained, and memory as the mechanism by which that information is retained. Learning and memory are dependent on synaptic plasticity that includes major changes in neurons and synapses (1). In 1984, Konorski presented the term “synaptic plasticity” for the first time to explain persistent and activity-dependent modifications in synaptic strength (2). Long-term potentiation (LTP) is described as the long-lasting activity-dependent enhancement of synaptic strength, which is commonly regarded as the closest neural model for explaining the molecular and cellular mechanisms involved in learning and memory (3). Long-term potentiation comprises three phases, including initial-LTP, early-LTP, and late-LTP, which occur consecutively over time to create what we call LTP. Initial-LTP (i.e., short-term potentiation) lasts for about 30 - 60 min and is a constant type of synaptic plasticity and dependent on the N-methyl-D-aspartate receptor (NMDAR). This phase of LTP does not require the activity of protein kinases. Early-LTP lasts for about 2 - 3 h and is not dependent on protein kinases’ activity. Finally, late-LTP persists for 5 - 6 h and requires gene expression and protein synthesis. Each phase is performed by different mediators and regulators (4). The high-frequency stimulation of presynaptic neurons leads to the secretion of neurotransmitters, chiefly glutamate. The binding of this neurotransmitter to the 2-amino-3-(3-hydroxy-5 methylisoxazol-4-yl) propanoic acid receptor (AMPAR) of postsynaptic neurons causes an influx of sodium into these neurons, leading to neuron depolarization. When the extent of depolarization is enough to eliminate the Mg2+-induced block of NMDAR, the influx of calcium into the postsynaptic neuron starts through this receptor. It has been well established that an optimal level of intracellular calcium-mediated NMDAR activation is essential for LTP induction. An increase in intracellular calcium concentration activates the signaling pathways that modify gene transcription and induce the synthesis of new proteins (5).
The induction of LTP needs the activation of many molecules and signaling cascades. Brain-derived neurotrophic factor (BDNF) is a key mediator that plays critical roles in the CNS, including promoting the survival and growth of neurons and inducing long-term synaptic plasticity (6-8). The mechanisms by which BDNF induces LTP are not fully understood. Substantial number of studies have suggested various roles for BDNF in the induction of hippocampal LTP. On the other hand, there is a relationship between BDNF and the neuronal mediators and pathways involved in LTP induction, including neurotransmitter systems, cellular receptors, and presynaptic and postsynaptic proteins. Thus, the present review primarily aimed to explain the impacts of BDNF on synaptic plasticity and LTP induction and then to elaborate the possible mechanisms responsible for these effects.
2. BDNF and LTP
Neurotrophins are a main group of signaling molecules widely investigated for their roles in promoting the survival, growth, and differentiation of neurons during development. Numerous studies have revealed that neurotrophins can also act as the regulators of synaptic plasticity. As one of the most important neurotrophic factors, BDNF is a 119-amino-acid basic peptide and has long been recognized as an anti-apoptotic factor extensively expressed in the brain. This factor shares important roles in the CNS, including neuronal maturation, synaptogenesis, synaptic plasticity, and hippocampal LTP induction (6, 7). Because of its essential role in neuronal development and function, changes in BDNF level have been described in a variety of psychiatric and neurological disorders (8, 9). Likewise, BDNF levels have been detected to decline in the CNS of patients with neurodegenerative disorders, including Huntington, Parkinson’s, and Alzheimer’s disease, neuropsychiatric disorders such as schizophrenia, and individuals with depressive-like behaviors (10-14).
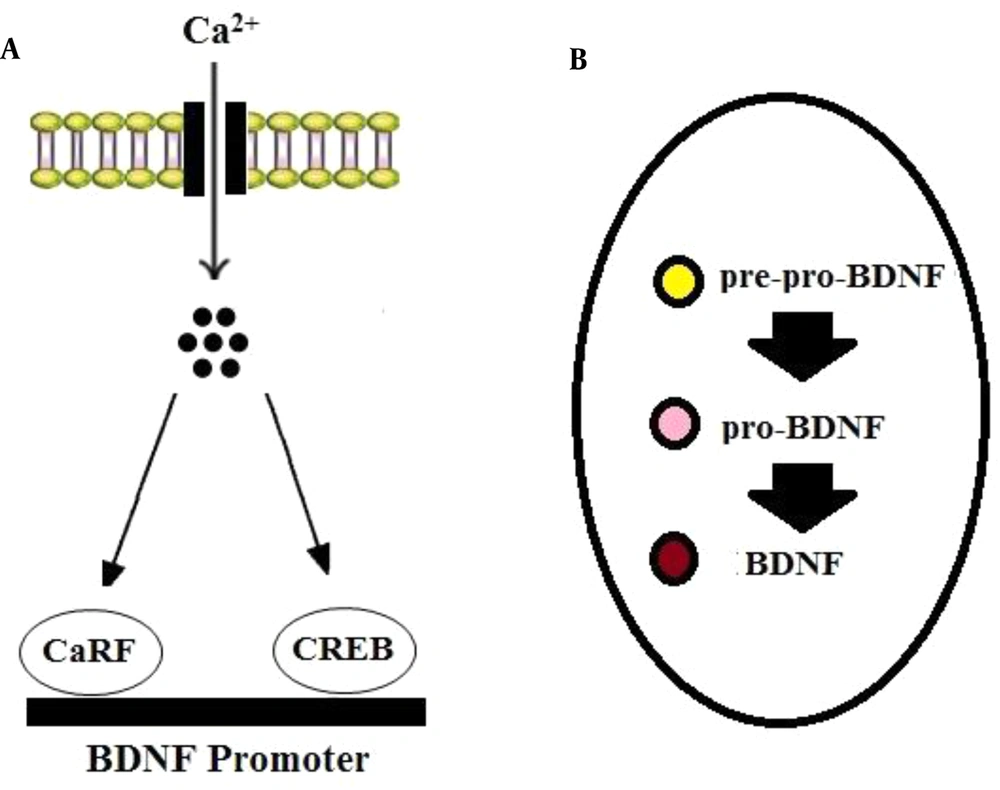
Brain-derived neurotrophic factor is produced in the soma of neurons and neuroglia and then is translocated to the terminals of pre-synapses (15, 16). The transcription of the gene encoding BDNF can be modulated by calcium influx. It has been established that calcium induces the binding of transcription factors, including cyclic AMP response element-binding protein (CREB) and calcium response factor (CaRF), to the bdnf gene’s promoters (17) (Figure 1A). In neurons, BDNF is synthesized as a precursor peptide known as pre-pro-BDNF that is cleaved into pro-BDNF (35 kDa). Then pro-BDNF can be cleaved by proteases into mature BDNF (mBDNF or BDNF: 14 kDa) (15, 16) (Figure 1B).
BDNF synthesis. BDNF mRNA transcription can be modulated via calcium influx through calcium channels. A, Ca2+ induces the binding of transcription factors, including CREB and CaRF, to BDNF promoters. B, In neurons, BDNF is first synthesized as pre-pro-BDNF in the endoplasmic reticulum and converted to pro-BDNF, which is then cleaved to mature BDNF.
Many studies propose that BDNF induces the complex neuronal signaling cascades responsible for the promotion of the cellular events involved in neuroplasticity and LTP induction (18, 19). For the first time, in the 1990s, it was reported that the treatment of hippocampus slices with BDNF enhanced early-LTP induced by theta-burst stimulation (20). Later, it was revealed that BDNF was involved in the early and late phases of LTP (21, 22). Consistent with these findings, De Vincent et al. reported that BDNF regulated the extent of LTP in the presynaptic neuron whereas in the postsynaptic neuron, it was involved in the maintenance of LTP (23). Furthermore, Messaoudi et al. assessed the impact of the intrahippocampal infusion of BDNF on synaptic plasticity and reported that the intrahippocampal microinjection of BDNF enhanced synaptic plasticity in the dentate gyrus of the hippocampus (24). Other studies also described a key role for BDNF in LTP induction, confirming that the knockout mice lacking the bdnf gene exhibited hippocampal LTP impairment (25-27).
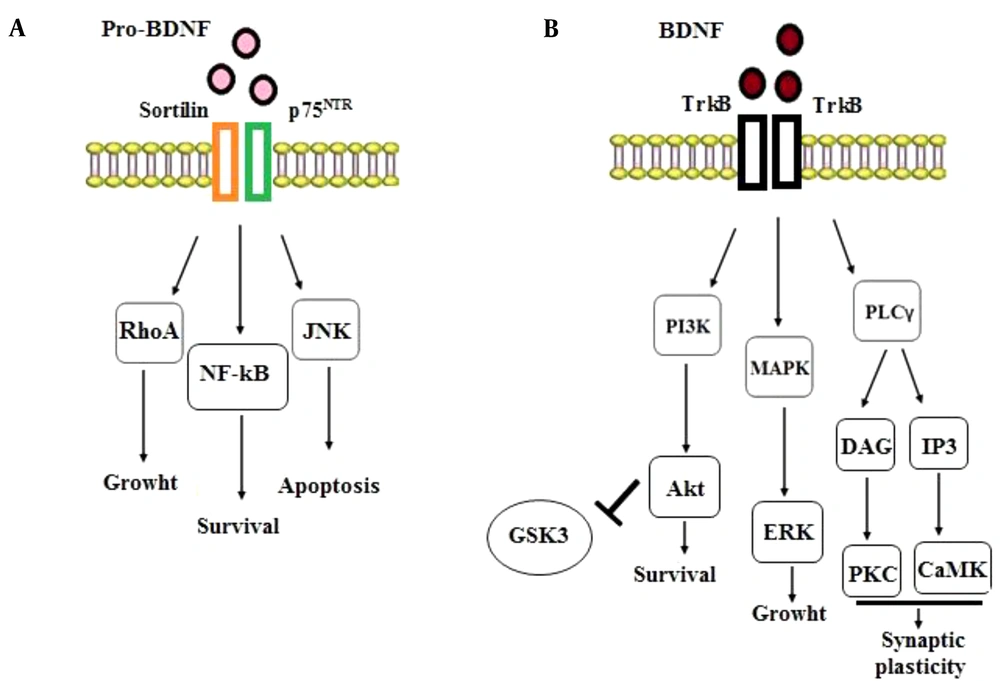
neurotrophic factors in response to physiological changes bind to to the tyrosine kinase (Trk) and p75 neurotrophin (p75NTR) receptors. Research findings propose that pro-BDNF and mature BDNF activate distinct cellular signaling pathways. Non-cleaved pro-BDNF leads to generally undesirable cellular events such as apoptosis and LTD induction through its binding to p75NTR; on the other hand, BDNF (i.e., mature BDNF) promotes positive events, including survival, growth, synaptic plasticity, and LTP induction through binding to TrkB receptors (28, 29). These findings suggest that pro-neurotrophins can elicit impacts opposite to mature-neurotrophins via binding to a different receptor (i.e., p75NTR) (30). In other words, the activation of TrkB receptors promotes cell survival, synaptogenesis, and synaptic plasticity whereas the engagement of p75 receptors leads to cell death and synaptic pruning (31). Consistent with these findings, LTD induction in the hippocampus has been reported to be dependent on the activation of p75 receptors (32). Overall, the binding of BDNF to the TrkB receptor mediates anti-apoptotic functions while pro-BDNF binding to p75NTR triggers apoptosis, a phenomenon that needs interaction with its co-receptor, sortilin. The pro-BDNF/p75NTR/sortilin complex begins the signaling pathways activating Ras-homolog gene family member A (RhoA), c-Jun amino terminal kinase (JNK), and nuclear factor kappa B (NF-ҡB) (33, 34). The activation of the RhoA-dependent signaling cascade regulates neuronal growth cone, development, and motility (34). The JNK-dependent signaling pathway stimulated by the proBDNF/p75NTR/sortilin complex induces neuronal apoptosis (33). Ultimately, the p75NTR-dependent activation of NF-ҡB triggers the processes stimulating neurons’ survival and maintaining their quantity during brain development (34) (Figure 2A).
The intracellular signaling pathways of BDNF-p75NTR and BDNF-TrkB. A, Binding of pro-BDNF to the p75NTR and sortilin receptors activates the signaling cascades related to RhoA, NF-ҡB, and JNK, stimulating the processes involved in neuronal development, survival, and programmed cell death; B, Binding of BDNF to the TrkB receptor initiates the MAPK, PI3K, and PLC γ pathways that promotes neuronal survival and growth and synaptic plasticity.
In addition, BDNF (mature BDNF) has a high affinity for binding to TrkB receptors, which subsequently induces the dimerization and autophosphorylation of this receptor, activating the downstream mitogen-activated protein kinase [MAPK, or Extracellular Signal Related Kinase (ERK)], phosphatidylinositol 3-kinase (PI3K), and phospholipase C-γ (PLC-γ) pathways (35, 36). The MAPK/ERK signaling pathway causes cell growth and differentiation, and the PLC-γ signaling route stimulates the inositol trisphosphate (IP3) receptor to release calcium, boosting calmodulin kinase (CamK) function and thus synaptic plasticity. Also, the PI3K signaling pathway activates protein kinase B (AKT), extending cell survival. Evidence from different studies suggests that the PI3K/Akt pathway is essential for axonal elongation. Furthermore, when the PI3K signaling pathway is activated, it leads to the inhibition of the downstream glycogen synthase kinase-3 (GSK-3), a serine/threonine kinase originally recognized as a regulator of glycogen metabolism (35, 37) and known to be extensively expressed throughout the CNS (38). On the other hand, GSK-3 has been shown to play an important role in NMDAR-dependent LTD at CA3–CA1 synapses of the hippocampus (39, 40). Previous studies reported that the local inactivation of GSK-3 was essential for axonal growth (41, 42). Moreover, other studies showed that various GSK-3 blockers could prevent the induction of LTD, when loaded into neuronal cells by a patch pipette (40, 43). Interestingly, it has been reported that BDNF inhibits GSK-3 by activating a PI3K-dependent cascade (44) (Figure 2B). Finally, these signaling pathways affect the cAMP response element-binding protein (CREB) transcription factor that can up-regulate the expression of relevant survival genes. It is notable that rapid effects on synapses and ion channels are dependent on PLCγ-mediated calcium release, and more long-lasting impacts, such as gene transcription, are downstream to the PI3K and MAPK signaling pathways. The activation of these pathways promotes neuronal survival and growth and neuroplasticity (8, 35, 36).
Dendrites are known to integrate synaptic inputs into neuronal cells, and their branching is associated with their representational capacity. Interestingly, the branching forms of dendritic trees are associated with the extent of the compartmentalization of inputs to neurons, and a stronger compartmentalization potential has been suggested to increase the representational capacity of the neuron, leading to a greater neuroplasticity capacity (45). The administration of diverse types of BDNF molecules mediates different morphological alterations. For example, the acute application of BDNF stimulated neurite elongation and spine head enlargement while its gradual application enhanced the branching of dendritic and filopodia-like spines (46). The effects of BDNF on cellular responses are also complex. For instance, the rapid perfusion of BDNF (i.e., acute exposure) increases synaptic transmission at neuromuscular synapses (47) while long-term (chronic) treatment with BDNF stimulates synapse maturation (48). A previous study found that the perfusion of BDNF in hippocampal slices induced a long-lasting improvement in basal-synaptic transmission (49). However, some studies have demonstrated that LTP, but not basal synaptic transmission, is facilitated in the hippocampal slices perfused with BDNF (50, 51). In support of these findings, Ji et al. reported that cellular responses to BDNF differed dramatically depending on how BDNF was delivered. In the neonatal slices of the hippocampus, slow BDNF perfusion (i.e., chronic exposure) facilitated the LTP induced by theta burst stimulation, without altering the baseline synaptic strength. In contrast, in adult hippocampus slices, the rapid perfusion of BDNF (acute) stimulated the synaptic growth necessary for the establishment of neuronal networks during development and induced structural and functional modifications in synapses (46). Kang et al. reported that the chronic or acute application of BDNF induced the long-lasting enhancement of basal synaptic transmission in the CA1 area of the hippocampus (49). However, some studies reported that slow or chronic BDNF perfusion did not induce such an enhancement (26, 50). On the other hand, in hippocampus slices, the rapid and slow increase of BDNF enhanced basal synaptic transmission and LTP induction, respectively (46). Overall, BDNF regulates an extensive range of cellular functions in the CNS, such as dendritic and axonal growth, survival of neurons, signal transmission in synapses, and LTP induction in the hippocampus.
3. BDNF and Neurotransmission
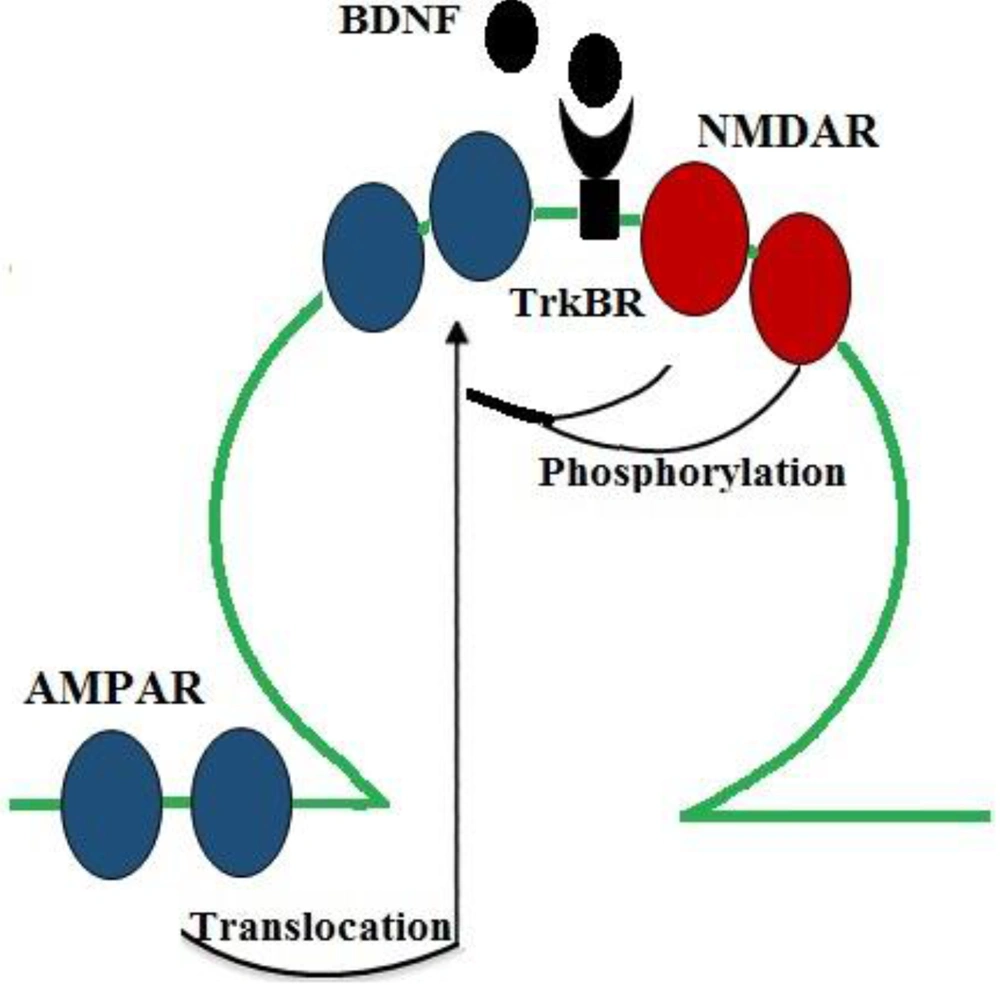
A great number of studies suggest that optimal brain function requires an intermediate range of neurotransmitters, and both high and low levels of neurotransmitters are involved in the pathophysiology of many mental diseases. Synaptic plasticity is commonly regulated by the release of various neurotransmitters from presynaptic neurons. Actually, dysregulated neurotransmitter systems can impair synaptic plasticity (52, 53). Some studies have reported that BDNF can regulate neurotransmission and LTP induction through both presynaptic and postsynaptic pathways. Consistent with these findings, the application of BDNF to cortical and hippocampal neuronal cultures and slice preparations induced excitatory neurotransmission and increased glutamate release (36, 49, 54, 55). Glutamate is one of the most important excitatory neurotransmitters in the CNS, and glutamate ionotropic receptors mediate most of excitatory neurotransmission. In fact, NMDAR and AMPAR are two types of ionotropic glutamate receptors (ligand-gated ion channels) that have critical roles in neuroplasticity and LTP induction. Glutamatergic synapses act mainly through AMPAR to generate rapid synaptic excitation, and NMDAR can also contribute to neurotransmission and plasticity (56). The activation of NMDAR increases calcium levels in the postsynaptic neuron and modulates the downstream signaling cascades involved in LTP induction (57). Considerable evidence suggests that BDNF promotes glutamatergic synaptic transmission in postsynaptic neurons by modulating NMDAR phosphorylation (58, 59). In line with these findings, it was reported that BDNF increased the phosphorylation of the NR1 and NR2B subunits of NMDAR (58) and enhanced the probability of the opening of NMDAR channels (54) to increase calcium influx (60). Moreover, AMPAR mediates most of the fast excitatory synaptic transmission in the brain of mammals. In the hippocampus, early-LTP maintenance is accompanied by an increase in AMPAR. According to some studies, changes in the number and type of the subunits of this receptor can impair synaptic plasticity. A considerable number of studies have also reported that BDNF modulates the surface expression of AMPAR through regulating its quick surface translocation to enhance excitatory transmission (61, 62) (Figure 3).
BDNF/TrkB actions on NMDAR and AMPAR, which mediate synaptic transmission in neurons. BDNF increases glutamatergic neurotransmission through increasing the probability of the opening of NMDAR (by promoting its phosphorylation) and through inducing the surface expression of AMPAR (by inducing the rapid translocation of its subunits to the surface) to enhance excitatory transmission.
Evidence suggests that a decrease in the level of monoamine neurotransmitters, including serotonin, dopamine and norepinephrine, impairs brain functions. Interestingly, BDNF regulates the activity of the dopaminergic, serotonergic, and noradrenergic systems (63, 64) and changes the secretion of neurotransmitters by altering protein expression in the presynaptic neurons responsible for releasing these mediators (65). Glutamate production in glutamatergic neurons is induced via a rise in the number of docked vesicles in the active zones of synapses (66). A previous study reported that the mice lacking BDNF showed a decline in the release of neurotransmitters in neuronal synapses, which was associated with a drop in the number of docked synaptic vesicles (67) and a reduction in the content of different presynaptic proteins, including synapsin, synaptophysin, and synaptobrevin, which are essential for vesicle docking and exocytosis in synaptic active zones (27). In line with these findings, Jovanovic et al. have reported that BDNF binds to the TrkB receptor and activates MAPK in synaptosome, inducing synapsin I phosphorylation at MAPK-dependent P-sites and the release of neurotransmitters (55).
Further evidence suggests that BDNF, in addition to influencing presynaptic proteins, alters the levels of postsynaptic proteins. As the most abundant postsynaptic protein, PSD-95 regulates different types of synaptic transmission, synapse structure and stability, and the formation and long-term stabilization of memory. The expression of PSD-95 enhances synapse maturation while the knockdown of this protein weakens synaptic strength and reduces spine density (68). Also, PSD-95-dependent protein complexes interact with NMDAR- and AMPAR-type glutamate receptors (69), which in the postsynaptic space can be vital for neuroplasticity and synaptic plasticity (70). Furthermore, the BDNF-TrkB pathway has an essential role in the development of synapses through the synaptic delivery of PSD-95 by vesicular transport (71) and its initial association with the membrane (71, 72).
Moreover, it appears that BDNF can affect synaptic plasticity and LTP induction via interaction with the sigma-1 receptor (Sig-1Rs) that is extensively distributed in different areas of the CNS, such as the prefrontal and parietal cortex, hypothalamus, and hippocampus (73). This receptor plays an important role in regulating different types of ion channels (74, 75), including voltage-gated ion channels that are critical for initiating and shaping action potentials (76) and NMDA-induced neuronal firing in the hippocampal CA3 region (77). The agonists of Sig-1Rs enhance synaptic plasticity, neuroplasticity, and memory functions (78, 79). It also has been reported that Sig-1Rs ligands affect memory functions via modulating NMDA-dependent LTP (75) and increasing BDNF level (80, 81). In a previous study, Malik et al. reported that the administration of a Sig-1Rs agonist stimulated the release of BDNF in rat primary cortical cultures (82), proposing that Sig-1Rs may facilitate BDNF secretion. Furthermore, Lever et al. reported that a Sig-1Rs agonist, cutamesine (SA4503), contributed to the trafficking, processing, and also release of BDNF from the neuronal B104 cell line (83). In agreement, the release of BDNF was shown to be inhibited by a Sig-1Rs antagonist (i.e., NE-100) (84). Overall, these findings confirmed a relationship between Sig-1Rs and BDNF, which can mediate synaptic plasticity and LTP induction.
Androgens have been suggested to exert profound effects on hippocampal structure and LTP induction (85, 86). According to some reports, it seems that the binding of androgens to androgenic receptors (ARs) induces a series of molecular mechanisms that activate BDNF via a TrkB receptor-dependent manner, finally resulting in enhanced synaptic plasticity and LTP induction. In a previous study, Salimi et al. reported a link between ARs and BDNF, mediated by calcium influx through Ca2+ channels (87). Therefore, the interaction between ARs and BDNF may affect neuroplasticity and LTP induction. In conclusion, BDNF is a neurotrophic factor that plays a fundamental role in the activation of cellular receptors and neurotransmitter systems and the induction of the presynaptic and postsynaptic proteins involved in synaptic responses. These properties make BDNF an essential modulator of the LTP process and synaptic plasticity.
4. BDNF and Free Radicals
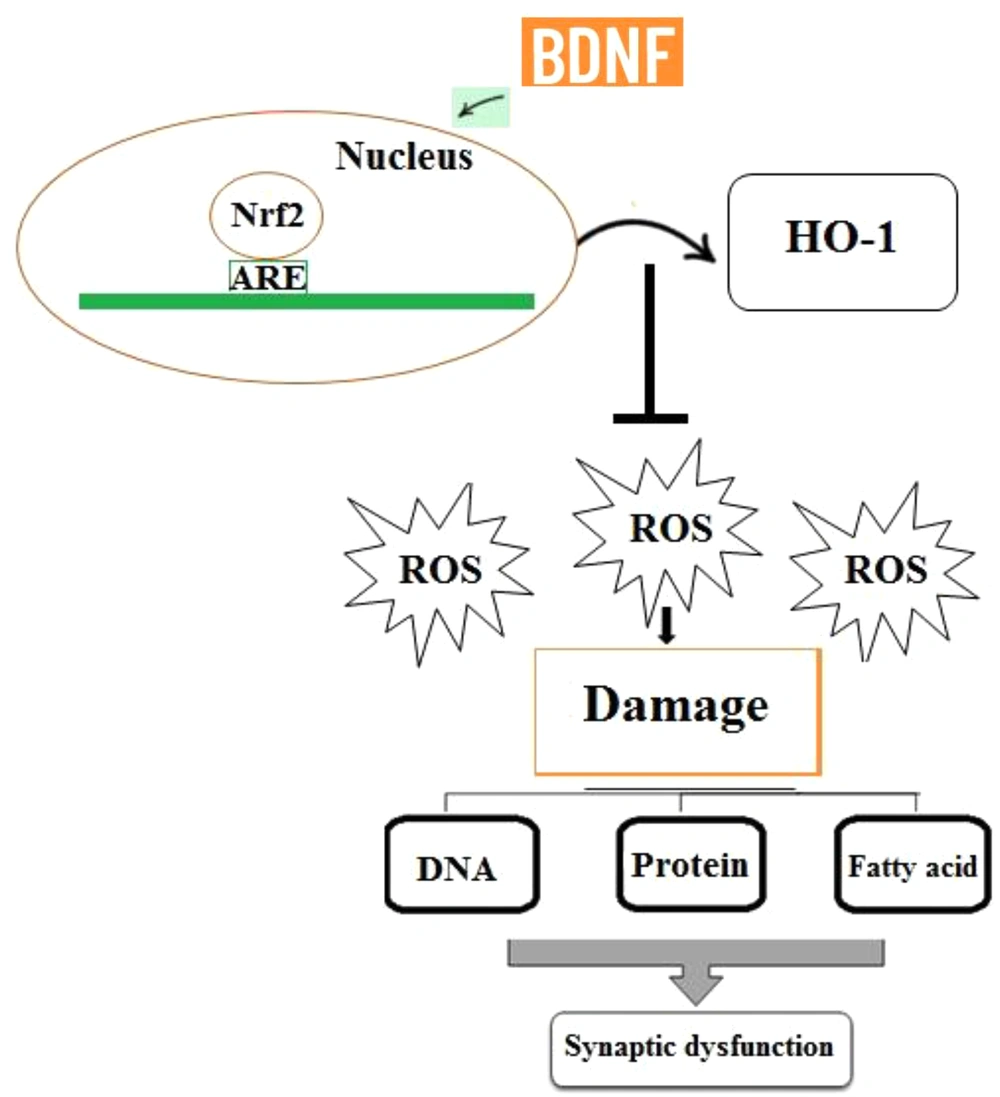
Reactive oxygen species (ROS) encompass the initial species formed by oxygen reduction (i.e., hydrogen peroxide (H2O2) or superoxide (O2-), as well as their secondary reactive products. These radicals are commonly generated in cells during metabolism, and the disruption of the balance between the formation of these radicals and the antioxidant capacity of cells can lead to oxidative stress. The increased production of ROS can pose a threat to cells by oxidizing proteins, inducing fatty acid peroxidation, and causing damage to nucleic acids. The brain is especially vulnerable to oxidative stress since it uses a large amount of oxygen and has a plentiful supply of lipids while it has a low antioxidant capacity (88, 89). Therefore, the accumulation of free radicals is very dangerous to the brain and can lead to memory impairment, synaptic dysfunction, neuronal death, and cognitive decay, and this is while antioxidants can abolish these effects (87). One of the most important cellular antioxidant defense mechanisms is the activation of the nuclear factor erythroid 2-related factor 2 (Nrf2) signaling pathway that regulates the expression of the genes whose protein products are involved in the detoxification and removal of reactive oxidants via conjugative reactions and increasing cells’ antioxidant capacity. Furthermore, Nrf2 is itself an important transcription factor regulating the expression of endogenous antioxidants, including heme oxygenase-1 (HO-1) (90). Indeed, Nrf2 deletion suppresses neurogenesis and leads to LTP impairment (91). Together, these findings reflect that the Nrf2 signaling pathway contributes to brain health and synaptic plasticity preservation in the hippocampus. According to the results of a previous study, there is a potential relationship between BDNF and Nrf2 activity. It has been reported that BDNF, as a homeostatic regulator of Nrf2 activity, induces its translocation into the nucleus in basic conditions, persistently activating antioxidant mechanisms (92) (Figure 4). In line with these findings, Bouvier et al. reported that low BDNF levels inhibited the activation of Nrf2-dependent endogenous antioxidant defense mechanisms, finally leading to persistent oxidative stress (92). Therefore, BDNF can enhance the antioxidant capacity of cells through inducing the translocation of Nrf2 into the nucleus. Overall, synaptic dysfunction and LTP impairment are associated with the accumulation of oxidative damage to macromolecules, and these negative effects can be reversed by the BDNF/Nrf2 pathway. In conclusion, BDNF shares a critical role in promoting Nrf2 nuclear translocation to preserve synaptic responses and LTP induction.
Increased production of ROS can cause damage to biomolecules including nucleic acids, fatty acids, and proteins. Therefore, ROS accumulation may be involved in synaptic dysfunction and LTP impairment. BDNF plays a critical role in promoting the nuclear translocation of Nrf2, which contributes to the expression of antioxidant genes and ROS removal.
4. Conclusions
As a member of the family of neurotrophins, BDNF has a fundamental role in the survival and differentiation of neurons during development and is involved in the plastic changes related to synaptic plasticity, as well as in learning and memory formation. This review aimed to discuss the effects of BDNF on LTP induction and also to describe the neuronal pathways involved in these effects of BDNF in the CNS. As one of the most important neurotrophins, BDNF induces complex neuronal signaling pathways that are essential for the cellular modifications underlying synaptic plasticity and LTP induction. Indeed, BDNF regulates a variety of functions in the CNS, such as dendritic and axonal growth, neuronal survival, synaptic signal transmission, activation of presynaptic and postsynaptic proteins, and LTP induction in the hippocampus. The brain is highly vulnerable to oxidative stress due to its high oxygen turnover that results in the formation of oxygen-related free radicals. In addition, the brain contains a high level of polyunsaturated fatty acids and a low antioxidant capacity compared to other organs and tissues. On the other hand, the Nrf2 transcriptional signaling pathway plays an essential role in regulating the genes encoding the proteins sharing critical roles in the detoxification and removal of free radicals. As described in this review, BDNF induces Nrf2 translocation into the nucleus in basic conditions. Thus, BDNF seems to promote its effects on neuroplasticity and LTP induction at least partly through activating antioxidant mechanisms and reducing free radical production. However, further research is needed to understand the interactions of BDNF-derived signaling with other mediators of synaptic