1. Background
Dental caries is known as a public health problem worldwide. It has been reported that the microbial factor is considered a main etiologic factor (1). The most known bacterium causing dental caries is Streptococcus mutans. Despite the leading role of cariogenic bacteria, extended cavity preparation is not a recommended treatment anymore (1). Nowadays, dental caries treatment is moving toward less invasive methods, such as the atraumatic restorative technique (ART) which emphasizes the conservative removal of the carious tissue and filling the cavities with fluoride-releasing materials (2, 3). A recent study has suggested that this method can induce significant improvement in oral and dental health, both for primary and permanent teeth, compared to the conventional methods in dentistry (4).
Among the important materials used in various treatments for caries are glass-ionomer cements (GICs), which have high biocompatibility and antimicrobial properties, making them highly desirable materials for use in dental treatments, especially in the ART method (5). Multiple studies have been carried out on enhancing the antimicrobial properties of GICs to inhibit the activity of the remaining bacteria beneath the restoration to prevent secondary caries and treatment failure. One of the methods of increasing antimicrobial properties of GICs is the addition of antimicrobial agents, such as metronidazole, ciprofloxacin, or cefaclor (6).
Yadiki et al. (7) examined the effect of adding chlorhexidine gluconate on the antibacterial properties of GICs against S. mutans. They claimed that the antibacterial effect of Fuji II and IX GICs containing chlorhexidine was higher than the control group. Ferreira et al. (6) also examined the effects of the addition of various antibiotics, such as metronidazole, ciprofloxacin, and cefaclor, on the antibacterial properties of GICs and showed that the addition of antibiotics to GICs increased their antibacterial activity and reduced the number or activity of cariogenic bacteria. Moreover, there has been a growing worldwide propensity toward using natural products for promoting oral health that can have a significant impact on preventing dental caries, given their pharmaceutical effects on the oral cavity (8).
Propolis is a natural resin substance made by bees. In addition, the reason for the different compositions of propolis is the specificity of plants and continents that have been used by bees in different areas (9). Propolis has various favorable properties, such as antimicrobial and antifungal. It has antimicrobial effects against a broad spectrum of bacteria, such as S. mutans, Staphylococcus aureus, Lactobacillus spp., and Enterococcus faecalis. Today, there are numerous products, including toothpastes and mouthwashes, which contain the proportions of propolis (10). The analysis of propolis effect on microorganisms showed that it could change their cellular membrane permeability and potential and adenosine triphosphate production and simultaneously decrease bacterial mobility (9).
A few studies have investigated the addition of propolis to GICs as an antimicrobial agent. Such studies have mainly focused on the effects of propolis on the antimicrobial properties of GICs and indicated that the addition of propolis to GICs increases its antimicrobial properties (11). The aforementioned studies did not particularly consider the effect of propolis on the mechanical properties of GICs (12); however, the success of restorative methods, especially the ART technique, is highly dependent on the mechanical properties of exploited restorative materials.
With this background in mind, this study examined the effect of the addition of propolis on the antimicrobial properties and flexural strength (which is one of the most paramount mechanical properties of restorative materials) of GICs. Different studies have been conducted on the ethanolic extract of propolis. However, since GICs are water-based, propolis aqueous extract was used in this study.
2. Methods
This study was approved by the Ethics Committee of Shahid Beheshti University of Medical Sciences (IR.SBMU.RIDS.REC.1395.237), Tehran, Iran, in 2019. In addition, the duration of the study setup was 9 months.
3.1. Preparation of Propolis Aqueous Extract
Propolis (Dr. Jahangir Pharmaceuticals Co., Lorestan, Iran) was frozen at a low temperature and then was milled using a micronized mill to achieve small propolis powder particles. The milled propolis (before becoming soft and sticky) was immediately added to boiling water with a ratio of 1: 2 of propolis and water in a particular container by an indirect heating system and a mixer. The solution was stirred for an hour. After 2 days, the sediments were separated from the aqueous extract solution with fabric filters and then with Watman filter paper with the largest pore size available. For the acceleration of the filtration process, a Büchner funnel and vacuum pump were used. For obtaining a purified solution without sediments, the solution was filtered by a filter press. The obtained extract had a yellowish-brown color.
3.2. Preparation of Experimental and Control Positive and Negative Groups
In this in vitro experimental study, a total number of 120 glass-ionomer disk samples were prepared in six groups of 20 as follows:
- Group 1: Fuji II (conventional) (GC Corp., Tokyo, Japan), a glass-ionomer control group.
- Group 2: Fuji IX (highly viscose) (GC Corp., Tokyo, Japan), a glass-ionomer control group.
- Group 3: Fuji II glass ionomer containing 25% propolis aqueous extract.
- Group 4: Fuji IX glass ionomer containing 25% propolis aqueous extract.
- Group 5: Fuji II glass ionomer containing 50% propolis aqueous extract.
- Group 6: Fuji IX glass ionomer containing 50% propolis aqueous extract.
For the control group, the powder and liquid of both brands of glass ionomers were mixed according to the manufacturers’ instructions. For increasing the accuracy of the test, the weight ratio of powder to liquid was measured with a digital scale (Acculab, AL-104, Germany) with a precision of 0.0001 g. Special mixing pads and a sterile metal spatula (L-7.5mm, D&P dental instruments, Iran) were used to mix the powder and the liquid to obtain a homogenous mixture.
In the glass-ionomer groups containing 25 and 50% propolis aqueous extract, the weight percentage calculated in the control group was used to mix the powder and the liquid, with the difference that 25 and 50% of the liquid weight of the glass ionomer in these groups were replaced by the propolis aqueous extract, respectively. First, the propolis aqueous extract was mixed with the glass-ionomer liquid, and then the powder was gradually added.
3.3. Evaluation of Antibacterial Properties
The S. mutans strain (PTCC1683), purchased from the Iranian Research Organization of Science and Technology, Tehran, Iran, was removed from the freezer and cultured in the Mueller-Hinton culture media at 37°C and incubated for 24 h at 37°C in an incubator (PECO, Iran). Subsequently, half McFarland turbidity (a concentration of 1.5 × 108 microorganisms per mL) was prepared from the bacterial suspension and cultured in Mueller-Hinton agar plates.
For the preparation of 20 disks for each of the six groups of this study, the mixtures of powder and liquid were put in plexiglass circular molds with a height of 2 mm and a diameter of 10 mm and properly condensed. Glass slides were applied on both surfaces to get a smooth surface. After setting, the specimens were steadily taken out of the molds and kept in an incubator (PECO, Iran) at 37°C for 24 h.
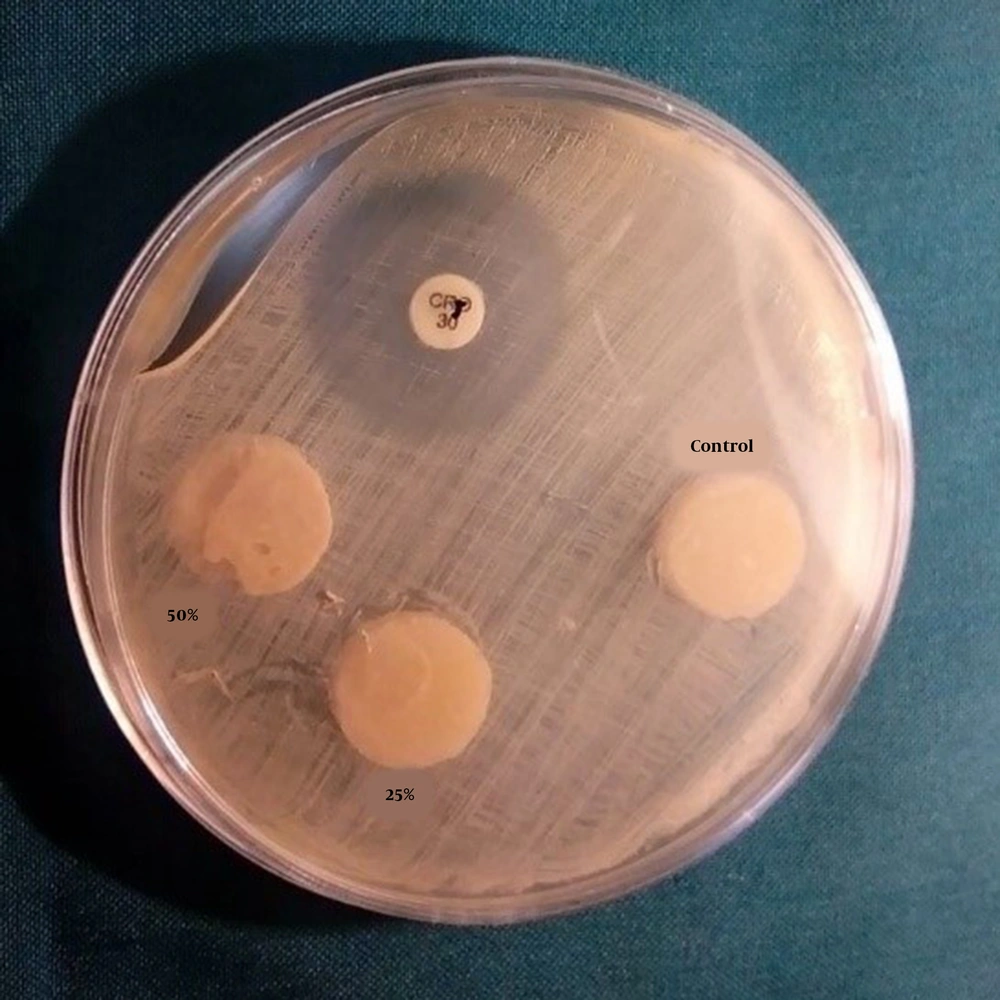
In each Mueller-Hinton agar plate, three disks (one disk from each group) and a ciprofloxacin 5 μg disk were placed. Moreover, on two prepared culture plates, three wells with Pasteur pipettes (Pasteur pipette, Pole Ideal Pars Co., Iran) were made with a diameter of 7 mm. In two wells of each plate, 30 mL of propolis aqueous extract and 30 μL of ciprofloxacin antibiotic with 0.5 mg concentration in one well were poured. After 24 h, the plates were removed from the incubator, and the diameter of the inhibition zone was measured in millimeters by a ruler from the back of each plate. For ensuring the accuracy of the results, this test was carried out three times (Figure 1).
3.4. Evaluation of Flexural Strength
For the measurement of the flexural strength of the six study groups, 15 samples from each group were examined according to the ISO 9917-21 standard. For the preparation of the samples, stainless steel molds with dimensions of 2 × 2 × 25 mm were used.
In each study group, after mixing the powder and the liquid, the obtained mixture was transferred to prefabricated cuboid molds and completely packed using a sterilized condenser (Dena Puya Co., Tehran, Iran) leaving no empty spaces in the molds. Afterward, a sterile glass slide was pressed on them to remove the excessive glass ionomer. The molds were then placed inside an incubator (PECO, Iran) at a temperature of 37°C and humidity of 100% for 2 min and 20 sec for the glass ionomer to undergo polymerization.
Then, the glass slides were steadily removed from the samples, and the samples were gently taken out of the molds. After removing the excess material from around each sample and ensuring that the samples were intact, the samples of each group were placed in a sterile container filled with distilled water and put in an incubator for 24 h at 37°C and 100% humidity for the final setting. After 24 h of incubation, the samples were gently removed from the containers, and their flexural strength was measured by a universal testing machine (ZwickRoell, Z020, Germany) with a crosshead speed of 1 mm per min. The maximum force and the breaking force were read from the device and recorded. The corresponding formula was used to calculate the flexural strength:
The Kolmogorov-Smirnov test and SPSS software (version 21.0) were used for statistical analyses and ensuring normal data distribution. One-way analysis of variance (ANOVA) was used for comparing the mean values of the groups, and Tukey’s HSD test was used for pairwise comparison of the groups.
3. Results
The evaluation of the effect of samples on the inhibition zone of S. mutans in the six study groups indicated that the mean inhibition zone diameter of the propolis aqueous extract, which was placed in the wells embedded on the culture, was 11 mm, and the mean inhibition zone around the wells containing ciprofloxacin was 15 mm. However, there was no bacterial growth inhibition zone around the disks in the glass-ionomer disk groups.
The normal distribution of the flexural strength data in the current study groups was evaluated using SPSS software (version 21.0). The p-values were considered higher than 0.05 for the dependent variables; therefore, the normal distribution of data was confirmed.
Based on the results of one-way ANOVA for evaluating the flexural strength of the Fuji II glass-ionomer control, 20 and 50% groups showed no significant differences among the three groups in this regard (P = 0.096). Furthermore, according to the results of one-way ANOVA for determining the flexural strength of the control, 25 and 50% groups of the Fuji IX glass ionomer showed no significant differences among the three groups (P = 0.905) (Table 1).
| Variables | n | Mean | Standard Deviation | Minimum | Maximum |
|---|---|---|---|---|---|
| Control-Fuji II | 5 | 11.75 | 1.43 | 10.42 | 13.40 |
| 25% Propolis-Fuji II | 5 | 9.90 | 1.13 | 8.42 | 11.03 |
| 50% Propolis-Fuji II | 5 | 10.87 | 1.09 | 9.34 | 12.15 |
| Control-Fuji IX | 5 | 16.72 | 2.38 | 14.42 | 20.69 |
| 25% Propolis-Fuji IX | 5 | 17.21 | 1.56 | 15.39 | 19.63 |
| 50% Propolis-Fuji IX | 5 | 17.19 | 1.84 | 14.86 | 19.91 |
Two-way comparisons of Tukey’s HSD test indicated a significant difference in the flexural strength values between the Fuji II and Fuji IX glass-ionomer groups containing propolis (P < 0.001). Accordingly, the groups containing 25 and 50% propolis of the Fuji II glass ionomer had the lowest flexural strength values without any significant differences (P = 0.713), and the groups containing 50 and 25% propolis of the Fuji IX glass ionomer had the highest flexural strength values without any significant differences (P = 1.000). Finally, yet importantly, the Fuji IX group had higher flexural strength values than the Fuji II group (P < 0.001) (Table 1).
4. Discussion
Nowadays, due to the increasing trend in using tooth-colored dental materials, such as GICs in restorations, especially in the ART technique, special attention has been paid to evaluating the antibacterial properties of such cements to reduce the risk of dental caries. In previous studies, the effect of adding different compounds, including chlorhexidine, calcium phosphate, and antibiotics, to GICs was evaluated to improve the antibacterial properties of these materials.
In recent years, the use of natural products for pharmaceutic and therapeutic purposes has undergone a substantial increase. One of such products is propolis, with numerous pharmaceutical features, such as antibacterial properties. Accordingly, propolis addition to GICs is expected to increase the anti-cariogenic properties of such cements (11). The results of the present study regarding the effects of propolis on antibacterial properties of GICs showed that although the propolis aqueous extract used in this study had antibacterial properties by itself, it did not show any antibacterial properties when added to GICs.
Topcuoglu et al. (11) claimed that the addition of propolis ethanol extract to the GIC enhanced its antibacterial properties in a way that the remaining S. mutans dry weight was the highest in the control group and the dry weight of S. mutans in the GIC groups containing 25% propolis was higher than the 50% propolis groups.
Hatunoglu et al. (13) investigated the effect of adding ethanolic propolis extract to GICs on its antibacterial properties using the Broth Agar method. The results showed that the growth rate of bacteria in the experimental group was lower than in the control group (13). Meneses et al. measured the antibacterial effect of adding propolis ethanol extract to GICs and observed that the antibacterial effect of Meron and Ketac Cem GICs increased proportional to the concentration of ethanolic propolis but turned upside down from day 1 to day 90 (14).
Among the reasons for different results between this study and the three aforementioned studies is the difference in the propolis solvent used in these studies. Water and ethanol were used in this study and other experimental studies, respectively. Regarding the importance of the propolis solvent type and its antibacterial activity, it can be referred to a study performed by Jafarzadeh Kashani et al., comparing the antibacterial effect of propolis aqueous and ethanol extract on various bacteria, such as S. mutans, Streptococcus salivarius, and Lactobacillus spp. The results indicated that the ethanolic extract affected a wider range of bacteria and its bactericidal feature was greater than that of propolis aqueous extract (15).
In this study, it was observed that although inhibition zones were present around the plate wells containing propolis aqueous extract, there was no inhibition zone around the glass-ionomer disks containing propolis, which were solid and completely polymerized. Therefore, it can be concluded that releasing antibacterial agents in liquid environments (i.e., mouthwashes) can be higher and more efficient than in solid environments, such as GICs. Yadiki et al. (7) argued that releasing chlorhexidine gluconate, an antimicrobial substance, from the Fuji IX GIC is difficult due to its hardness and viscosity.
The evaluation of adding the effect of different concentrations of propolis aqueous extract on the flexural strength of Fuji II and IX GICs showed no significant difference between the experimental and control groups. However, the flexural strength of the Fuji IX glass ionomer containing different propolis concentrations was significantly higher than that of the Fuji II glass ionomer (P < 0.0001).
Troca et al. investigated the effect of adding green propolis on the mechanical properties of GICs, such as diametral tensile strength. Troca et al. showed that, except in the Chemflex type, which showed reduced tensile strength, the strength of other propolis-containing GICs did not have any significant differences in comparison to that reported for the control group (16).
Subramaniam et al.’s study (17) was conducted on the effect of adding propolis on the compressive strength and solubility of GICs. The results demonstrated that adding propolis to the GIC reduced compressive strength and increased the solubility of propolis-containing samples, compared to those reported for the control group. It appears that propolis reduces water dispersion into the GIC by forming bonds with the resin networks; however, the presence of water is essential for the first stage of GICs’ polymerization. Furthermore, surface layer decomposition can be a substantial problem, leading to the saturation of ions and glass particles (18).
This study also showed greater flexural strength of the Fuji IX glass ionomer, compared to that reported for the Fuji II type. Frankenburger et al. (19) reported that the Fuji IX glass ionomer is more rapidly set and has higher viscosity due to smaller glass particles. Therefore, the aforementioned cements have higher flexural strength and resistance to abrasion.
One of the limitations of this study is the inability to evaluate the effect of adding propolis aqueous extract with higher concentrations due to laboratory constraints. Moreover, because laboratory conditions are not entirely similar to oral cavity conditions, the results cannot be generalized to clinical conditions. Furthermore, due to the brownish color of propolis, its addition to the glass ionomer causes undesirable color changes in the glass ionomer, which is of importance in esthetics, especially at high concentrations.
4.1. Conclusion
Despite the antibacterial effects of propolis aqueous extract, the addition of 25 and 50% concentrations of this extract did not enhance the antibacterial properties of Fuji II and Fuji IX GICs against S. mutans. Moreover, adding 25 and 50% concentrations of propolis aqueous extract did not have a negative effect on the flexural strength of the aforementioned GICs.
4.2. Limitations and Suggestions
The simulation of the present study was not precisely adapted to the oral environment; therefore, further studies can be performed in similar oral conditions.