1. Context
Practicing in the emergency department (ED) can include a variety of painful and unpleasant diagnostic or therapeutic procedures. Procedural sedation (PS), which means reducing the level of patient consciousness to facilitate the procedures in the emergency room, can provide more comfort for the patient and help emergency physicians in painful procedures (1, 2). Minimal effect on respiration and hemodynamic stability is desired by emergency physicians in selecting a sedative agent (3).
The agonists of alpha-2 adrenergic receptors have been shown to modulate arousal and wakefulness. Dexmedetomidine is a highly selective and potent alpha-2 adrenergic agonist with a dose-dependent effect, ranging from minimal to deep sedation (4, 5). Dexmedetomidine acts on presynaptic alpha-2 receptors, reduces the activity of the sympathetic nervous system, and causes profound sedation. It can also decrease blood pressure and heart rate by a reduction in the level of norepinephrine; however, respiratory depression is not a common side effect (6, 7). Due to its short half-life and low respiratory effects, dexmedetomidine can be a reasonable choice for PS in the ED.
Although dexmedetomidine was investigated in many trials for use in intensive care unit settings or induction of hypnosis and anesthesia in operating rooms, its role as a sedative agent for PS in the ED has not been well studied. Some clinical trials were conducted to study this issue; nevertheless, neither its effectiveness as a sedative agent in the ED nor its safety in this regard has not been systematically reviewed before. Therefore, the present study investigated the role of dexmedetomidine in PS in ED based on a systematic review of the current literature.
2. Methods
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.
2.1. Research Question
The research questions of this study examined the effectiveness and safety of dexmedetomidine in comparison to those of other sedative agents in ED patients. Population (P): Patients referred to the ED; Intervention (I): Dexmedetomidine; Control (C): Other sedatives; Outcome (O): Dexmedetomidine effectiveness and safety for PS.
2.2. Literature Search
In this study, several databases, including PubMed, Embase, Ovid, ProQuest, Scopus, Web of Science, and Cochrane Library, were systemically searched since 1999 up to November 30, 2020. In addition, manual searching of databases, such as Google Scholar, was conducted. The reference lists of all included studies were checked for any potential additional publications. The following search strategy was used for the PubMed database:
((("Dexmedetomidine"[Mesh]) OR ((((Dexmedetomidine[Text Word]) OR MPV-1440[Text Word]) OR MPV 1440[Text Word]) OR MPV1440[Text Word]))) AND (((("Emergency Service, Hospital"[Mesh]) OR (((((((((((((((("Hospital Emergency Services"[Text Word]) OR "Emergency Department"[Text Word]) OR "Emergency Departments"[Text Word]) OR "Emergency Hospital Service"[Text Word]) OR "Emergency Hospital Services"[Text Word]) OR "Emergency Unit"[Text Word]) OR "Emergency Units"[Text Word]) OR "Emergency Ward"[Text Word]) OR "Emergency Wards"[Text Word]) OR "Hospital Emergency Service"[Text Word]) OR "Hospital Service Emergency"[Text Word]) OR "Hospital Service Emergencies"[Text Word]) OR "Emergency Room"[Text Word]) OR "Emergency Rooms"[Text Word]) OR "Emergency Outpatient Unit"[Text Word]) OR "Emergency Outpatient Units"[Text Word]))) OR (("Emergencies"[Mesh]) OR ((Emergency[Text Word]) OR Emergencies[Text Word]))
“Emergency Department” AND “Dexmedetomidine” AND “Sedation” were used for searching Cochrane Library regarding relevant reviews.
2.3. Study Selection
The inclusion criteria for considering a study eligible to enroll in this review were as follows: (1) randomized clinical trials; (2) studies performed on ED patients; (3) use of dexmedetomidine for PS; (4) studies in the English language; (5) studies with available full-text.
We excluded the articles in any language other than English, low-quality articles, articles which were not clinical trials, studies conducted in any department other than the ED, and animal studies. Studies were excluded if they were duplicate publications, reviews, editorials, abstracts, commentaries, and case reports.
2.4. Data Extraction
Two authors (SP and SM) independently searched and selected eligible studies by screening the titles and abstracts. A third author (HS) was consulted in case of any disagreement. The Joanna Briggs Institute (JBI) checklist was used for the assessment of the risk of bias in enrolled articles. The JBI checklist is a tool for the evaluation of the quality of articles and includes an assessment of randomization procedure, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, and other possible biases. A critical appraisal of the articles was carried out by two authors. Discrepancies in evaluations were solved by consulting a third investigator.
3. Results
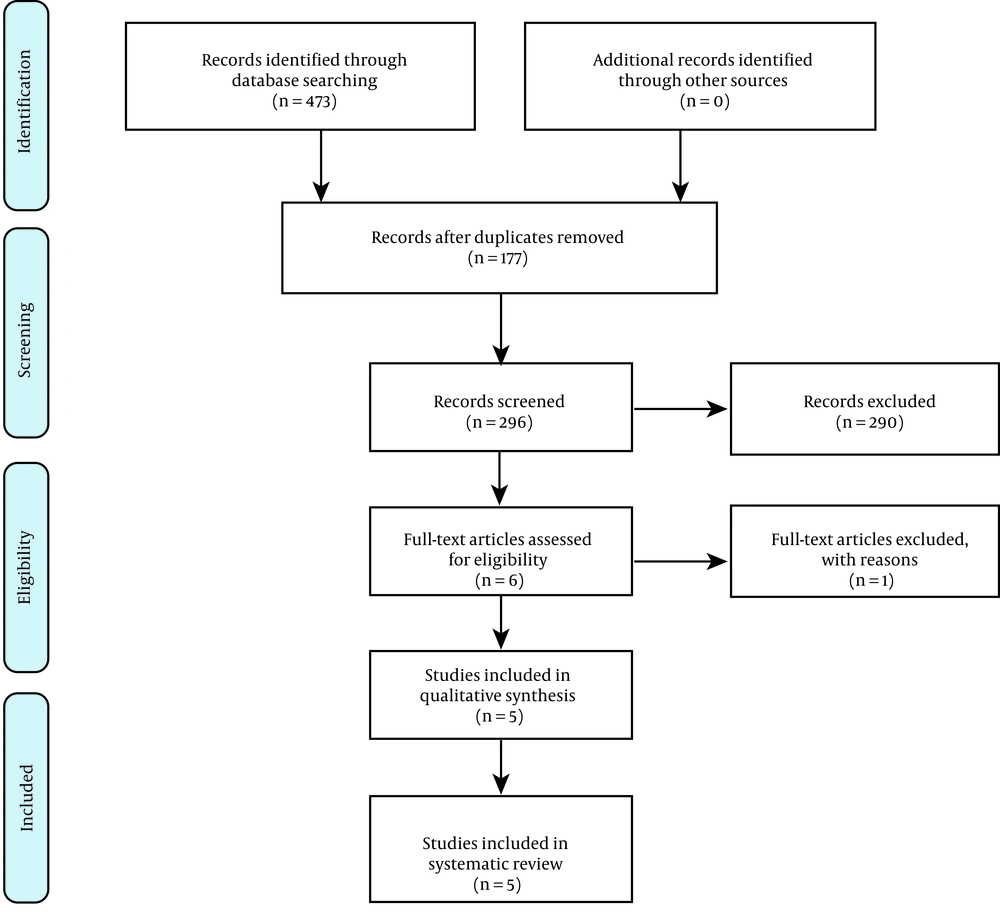
A total of 473 articles were identified after this systematic search, among which 177 studies were duplicates. Moreover, 290 records were identified as non-relevant to the study subject. One study was excluded due to its low strength and poor methodology. Figure 1 shows the flowchart of the identified and enrolled articles in this study. Finally, five studies fulfilled the eligibility criteria to enter this review. Table 1 shows the characteristics of the enrolled studies. Table 2 shows the assessment of the risk of bias based on the JBI checklist. The selected studies had a low risk of bias, as shown in Table 2. These studies were published within 2015 to 2020. Two studies were performed on the pediatric population. One study used dexmedetomidine in combination with fentanyl, and four studies used dexmedetomidine as a single medication. The route of drug administration was intravenous (IV) in three studies, intranasal (IN) in one study, and intramuscular (IM) in another study.
| Author | Year | Country | Study Type | Blinding Type | Sample Size | Group A | Group B | Initial Dose of Dexmedetomidine | Initial Dose of Group B | Population | Method | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group A | Group B | |||||||||||
| Neville et al. (8) | 2016 | USA | Randomized double-blind clinical trial | Double | 20 | 18 | Dexmedetomidine | Midazolam | 2 µg/kg | 0.4 mg/kg | Pediatric | IN |
| Porozan et al. (9) | 2019 | Iran | Randomized double-blind clinical trial | Double | 47 | 47 | Dexmedetomidine | Ketamin | 3 µg/kg | 4 mg/kg | Pediatric | IM |
| Masoumi et al. (10) | 2019 | Iran | Randomized double-blind clinical trial | Double | 30 | 30 | Dexmedetomidine | Midazolam-fentanyl | 1 µg/kg | 0.05 mg/kg midazolam combination with 1 µg/kg midazolam-fentanyl | Adult | IV |
| Kamali et al. (11) | 2018 | Iran | Randomized double-blind clinical trial | Double | 57 | 57 | Dexmedetomidine | Propofol | 0.4 µg/kg | 1 to 1.5 mg/mg | Pediatric | IV |
| Arhami Dolatabadi et al. (3) | 2018 | Iran | Randomized single-blind clinical trial | Single | 40 | 40 | Dexmedetomidine-fentanyl | Midazolam-fentanyl | 1 µg/kg | 0.01 mg/kg | Adult | IV |
Abbreviations: IN, intranasal; IM, intramuscular; IV, intravenous.
| Author | Was Proper Randomization Used for Assignment of Participants to Treatment Groups? | Was Allocation to Treatment Groups Concealed? | Were Treatment Groups Similar at the Baseline? | Were Participants Blind to Treatment Assignment? | Were Those Delivering Treatment Blind to Treatment Assignment? | Were Outcomes Assessors Blind to Treatment Assignment? | Were Treatment Groups Treated Identically Other Than the Intervention of Interest? | Was Follow-up Complete, and If Not, Were Differences Between Groups in Terms of Their Follow-up Adequately Analyzed? | Were Participants Analyzed in Groups to Which They Were Randomized? | Were Outcomes Similarly Measured for Treatment Groups? | Were Outcomes Measured in a Reliable Way? | Was Appropriate Statistical Analysis Used? | Was the Trial Design Appropriate? |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neville et al. (8) | ? | + | + | + | + | + | + | + | + | + | + | + | + |
| Arhami Dolatabadi et al. (3) | ? | _ | + | + | _ | _ | + | + | + | + | + | + | + |
| Porosan et al. (9) | _ | _ | + | + | + | + | + | + | + | + | + | + | + |
| Kamali et al. (11) | ? | _ | + | + | + | + | + | + | + | + | + | + | + |
| Masoumi et al. (10) | ? | ? | + | + | + | + | + | + | + | + | + | + | + |
Table 3 shows the grading of recommendations, assessment, development, and evaluation of the enrolled studies, evaluating the strength of the evidence in each study. Masoumi et al. (10) studied dexmedetomidine versus a mixture of midazolam-fentanyl in PS for anterior shoulder dislocation reduction. A double-blind randomized clinical trial investigated 60 patients in two groups. A group received dexmedetomidine (1 μg/kg loading dose followed by 0.2 μg/kg/h for 10 min), and the other group received IV midazolam (0.05 mg/kg) and midazolam-fentanyl (1 μg/kg) for 10 min. Masoumi et al. observed a faster and higher level of sedation for dexmedetomidine in comparison to that reported for midazolam-fentanyl (10).
| Study | Recommendation | Grade |
|---|---|---|
| Neville et al., 2016 (8) | Intranasal administration of dexmedetomidine and midazolam were similarly performed in terms of anxiolysis for laceration repair. | B |
| The patients receiving intranasal dexmedetomidine had less anxiety at the time of positioning for the procedure than those receiving midazolam. | A | |
| Intranasal dexmedetomidine is an alternative anxiolytic medication to intranasal midazolam for pediatric laceration repairs, performing similarly in the present study, except that patients receiving dexmedetomidine had less anxiety at the time of positioning for the procedure. | B | |
| Arhami Dolatabadi et al., 2018 (3) | The mean pain score at the time of reduction was not significantly different between the two groups from a clinical point of view. | B |
| The dexmedetomidine group had a significantly shorter time to recovery (P < 0.001). | A | |
| The absolute risk increase rate of treatment failure in case of using dexmedetomidine instead of midazolam was 17.50% (95% CI: 4.19-30.81), and the number needed to harm was 6.00 (95% CI: 3.20-23.80). | B | |
| Although the combination of dexmedetomidine-fentanyl had a shorter time to recovery, compared to midazolam-fentanyl, for the induction of sedation and analgesia, the treatment failure rate in case of using dexmedetomidine with 1 µg/kg increased 17.5%. About one out of each six patients also needed a rescue dose. | B | |
| Porozan et al., 2019 (9) | The mean time of sedation onset in the ketamine group was significantly lower, compared to that of the dexmedetomidine group (P < 0.001). | B |
| The mean duration of sedation effect in the ketamine group was significantly lower than that of the dexmedetomidine group. | A | |
| There was no significant difference in the mean discharge time between the two groups. | B | |
| Although dexmedetomidine had a slower onset of effect and a longer duration of effect, compared to ketamine, due to its lower side effects, it can be an appropriate alternative to commonly used sedative medications. The combination of ketamine and dexmedetomidine can increase effectiveness and reduce side effects, requiring performing further studies. | B | |
| Kamali et al., 2018 (11) | Compared to group dexmedetomidine, the propofol group significantly showed increases in mean arterial pressure and systolic blood pressure at all times and immediately after the endotracheal intubation. | A |
| Moreover, the mean diastolic blood pressure changes due to tracheal intubation in the propofol group were significantly higher than those of the dexmedetomidine group immediately after the intubation. | A | |
| Furthermore, the mean heart rate changes immediately and 5 min after tracheal intubation were significantly higher in the propofol group. | A | |
| Changes in oxygen saturation in the two groups did not have a significant difference. | B | |
| The benefits of dexmedetomidine were higher than those of propofol in hemodynamic stability because propofol was associated with more variability in systolic/diastolic blood pressure, heart rate, and mean arterial pressure after endotracheal intubation. | A | |
| Masoumi et al., 2019 (10) | The time to reach the desired sedation (reach to a minimum of visual analog scale score and moderate sedation) in the dexmedetomidine group was lower than that of the midazolam-fentanyl group (P = 0.001). | A |
| Moreover, the visual analog scale mean scores in the midazolam-fentanyl and dexmedetomidine groups were 3.3 ± 1.24 and 2.57 ± 0.9, respectively. | A | |
| There was no significant difference between the times to reach the desired level of analgesia. | B | |
| Dexmedetomidine provides a higher level of analgesia than midazolam-fentanyl. Moreover, it was also shown that dexmedetomidine causes faster procedural sedation than midazolam-fentanyl. | A |
Arhami Dolatabadi et al. studied a mixture of dexmedetomidine and fentanyl for PS in distal radius fracture reduction (1 mcg/kg dexmedetomidine and 3 mcg/kg fentanyl). They compared this mixture with the midazolam-fentanyl mixture (10 mcg/kg midazolam and 3 mcg/kg fentanyl) in a randomized clinical trial. The aforementioned study examined 80 patients in two groups, namely groups 1 and 2 receiving dexmedetomidine-fentanyl and midazolam-fentanyl, respectively. Arhami Dolatabadi et al. mentioned that they did not perform double-dummy blinding due to different administration methods for two medication protocols. They observed a shorter recovery time for dexmedetomidine-fentanyl mixture in comparison to that of midazolam-fentanyl; however, this method of PS had a higher failure rate, and one patient out of each six cases needed a rescue dose. No specific side effect was noticed in each group except transient bradycardia in the dexmedetomidine group, which was resolved after slowing the infusion rate (3).
A double-blind randomized control trial performed by Kamali et al. compared dexmedetomidine with propofol in controlling hemodynamic responses after intubation in the ED. After pretreatment with fentanyl and lidocaine, two groups of patients, with 57 patients in each group, received one of the medication protocols, including a group receiving 0.4 μg/kg dexmedetomidine and the other group receiving g 1 - 1.5 mg/kg/h propofol as an induction agent. The results showed better hemodynamic stability in the dexmedetomidine group (11).
Neville et al. performed a double-blinded control trial on the pediatric population to compare the anxiolytic effect of IN dexmedetomidine and IN midazolam on wound management. The aforementioned study investigated 40 patients in two groups, including 20 and 18 patients receiving 2 mcg/kg of IN dexmedetomidine and 0.4 mg/kg of IN midazolam, respectively. The anxiety at the time of positioning for laceration repair was the primary outcome of the study. Neville et al. reported dexmedetomidine as effective as midazolam. No significant side effect was also observed in the groups (8).
Porozan et al. studied IM dexmedetomidine and IN ketamine for PS in children undergoing computed tomography. In a double-blinded clinical trial, 94 children were studied in two groups. Dexmedetomidine was observed as a safe alternative to ketamine for PS in children, although it has a slower onset of action and a longer duration of sedation (9).
4. Discussion
This systematic review investigated the role of dexmedetomidine in PS in ED. Dexmedetomidine, due to its hemodynamic profile, is widely used for PS (12); nevertheless, its use in emergency settings has been less studied. The present study aimed to compare the efficacy of dexmedetomidine and other sedative agents in emergency patients by systematically reviewing the current studies. This systematic review suggested that dexmedetomidine in either IV, IM, or IN route of administration could be a safe option for PS in the ED and has satisfactory effectiveness, although it is required to perform further studies to draw a firmer conclusion.
In none of the studies, no significant adverse effect was reported for dexmedetomidine. Only in one study with the IV route of administration, bradycardia occurred which was transient and was resolved by a slower infusion rate (3). Similar to the current study, another systematic review comparing dexmedetomidine and midazolam in the adult population observed dexmedetomidine as a safe alternative to midazolam (5). Li et al. performed a systematic review on the pediatric population comparing IN dexmedetomidine with oral chloral hydrate. They also reported a lower blood pressure and heart rate in dexmedetomidine receivers; nonetheless, Li et al. concluded that dexmedetomidine is a safe alternative to oral chloral hydrate (13).
A significant chance of failure in PS was reported in two studies. Porozan et al. reported significant failure in the dexmedetomidine group requiring a second administration of the drug. The route of administration was IM in the aforementioned study (9). The combination of dexmedetomidine with fentanyl also had a higher failure chance compared to midazolam-fentanyl IV administration (3).
4.1. Limitations
Due to the small number of the included studies in this review, performing a meta-analysis was not possible. The reviewed studies also had different routes of administration and doses and were conducted both in adult and pediatric populations.
4.2. Conclusions
Although some studies reported dexmedetomidine as a safe and effective drug in PS in the ED, there are not sufficient data to conclude about its role in this regard. Therefore, it is required to carry out further clinical trials with a larger sample size and strong methodology to investigate the role of dexmedetomidine in PS.
4.3. Availability of Data and Materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.