1. Background
Despite global legislative and regulatory efforts to enhance food safety, mycotoxins remain a recurring challenge (1) in staple foods such as maize and other locally produced cereals (2) in South Africa. The country is regarded as “a food secure nation”, however, Statistics South Africa (3) and the South African National Health and Nutrition Examination Survey (SANHANES-1) indicated a high level of food insecurity at household level (4). This situation is common with maize (Zea mays), the main staple household food and animal feed (5) in the country. Like many other food commodities, maize produced for human and animal consumption should be safe and supplied in sufficient quantities (1). Therefore, fungal infections and the production of associated secondary metabolites, e.g., mycotoxins, have profound effects on maize farming and downstream activities (1).
Mycotoxin-producing fungi belong mainly to the genera Aspergillus, Alternaria, Claviceps, Fusarium, Penicillium, and Stachybotrys, which are common contaminants of maize (2, 6). Some mycotoxins are immunosuppressive, carcinogenic, nephrotoxic, and neurotoxic in both animals and humans (1, 7-9). For example, according to Grace et al. (10), over 9000 cases of liver cancer per annum, stunting, and immune suppressions in children (11) have been associated with AF exposures. Primarily, mycotoxin effects vary based on the type of mycotoxin, level, and duration of exposure as well as age, sex, and health status of exposed individuals (12). Direct exposure is mainly through ingestion of contaminated food or inhalation of fungal spores and dust particles containing the toxin (13). Indirectly, mycotoxins are consumed via the dermal or maternal (umbilical cord and breast milk) route (13), and cereals together with nuts are reported as the major source of dietary exposure to mycotoxins (14).
Several outbreaks of mycotoxicosis among humans and animals have been reported in South Africa. In 2001, in a primary school nutrition programme (PSNP) in the Eastern Cape, children were hospitalized after consumption of peanut butter highly contaminated with aflatoxins (AFs) (15). In another mycotoxin-related disease outbreak, Arnot et al. (16) reported the death of over 220 dogs in the Gauteng province after the consumption of pet food contaminated with high concentrations of AF. Similarly, nephropathy in pigs was reported in South Africa, with a combination of ochratoxin A (OTA) and FB1 having concentration levels of 67 - 251 µg/kg and 5021 - 5289 µg/kg, respectively, as well as penicillic acid (PA) at 149 - 251 µg/kg (17). Mycotoxins have, therefore, been considered as significant contaminants of food, which carry a high burden (both in morbidity and mortality) in South Africa (17-19).
Studies conducted in South Africa showed that FB is the most common mycotoxin group affecting maize and maize products (20-23). Other mycotoxins, including AFs, OTs, zearalenone (ZEA), and trichothecenes, are also common, though recovered at low concentrations (24, 25). Favorable climatic conditions are likely to increase fungal colonization that causes increased quantities of mycotoxins in maize and other foodstuffs (2, 26), which in turn exacerbate health and economic consequences.
2. Objectives
This scoping review summarizes available data on mycotoxin contamination of maize, maize-based products, and other cereals. The aim is for understanding, informing, and enhancing protection against fungal contamination in South Africa, responsible for producing these products in sub-Saharan Africa.
3. Method
3.1. Scoping Review Method
Following the Arksey and O'Malley (27) method, a scoping review was conducted from April 2014 to July 2020 to collect evidence of different mycotoxins contaminations on maize and other cereal products as well as mycotoxin exposure among humans in South Africa. Although some of these secondary metabolites have been reported in South Africa, challenges were encountered in retrieving related articles for this review. For example, in cases where the cost of retrieving articles was a limitation, attempts were made to contact corresponding authors for assistance. The search protocol for this study was restricted to both published and unpublished articles and reports between 1980 to 2020. We selected a relatively long period (i.e. 40 years) in order to capture a wide range of literature on mycotoxins in South Africa.
3.2. Identification and Extraction of Articles
The following key guides were used to identify and extract related articles (27); “the research question, relevant studies, selection of relevant studies, final collation, summarizing, and reporting of the results”. Based on this methodology, the main research question for this review was “are South Africans exposed to high levels of mycotoxins, and if so, why?”. Then, keywords were generated from an initial search of this question against databases. This process was intended to both facilitate and increase the sensitivity of the search with these key phrases, which included “mycotoxins and food safety in South Africa”, “mycotoxin exposure in South Africa”, and “mycotoxicoses and mycotoxins in South Africa”. Articles and reports for this scoping review were all published in English and used as abstracts, full publications, dissertation, thesis, case studies, reviews, books, or book chapters. An excel spreadsheet was created and used to collate and screen for relevant studies on mycotoxins in maize and other cereal. Data related to information on “maize and other cereal products associated with mycotoxin contamination from 1980 to 2020 in South Africa as well as quantitative measurements of different types of mycotoxins” were collected. Article review and data collection were performed by postgraduate students who were trained on how to extract and critic relevant articles to identify research gaps.
3.3. Data Analysis
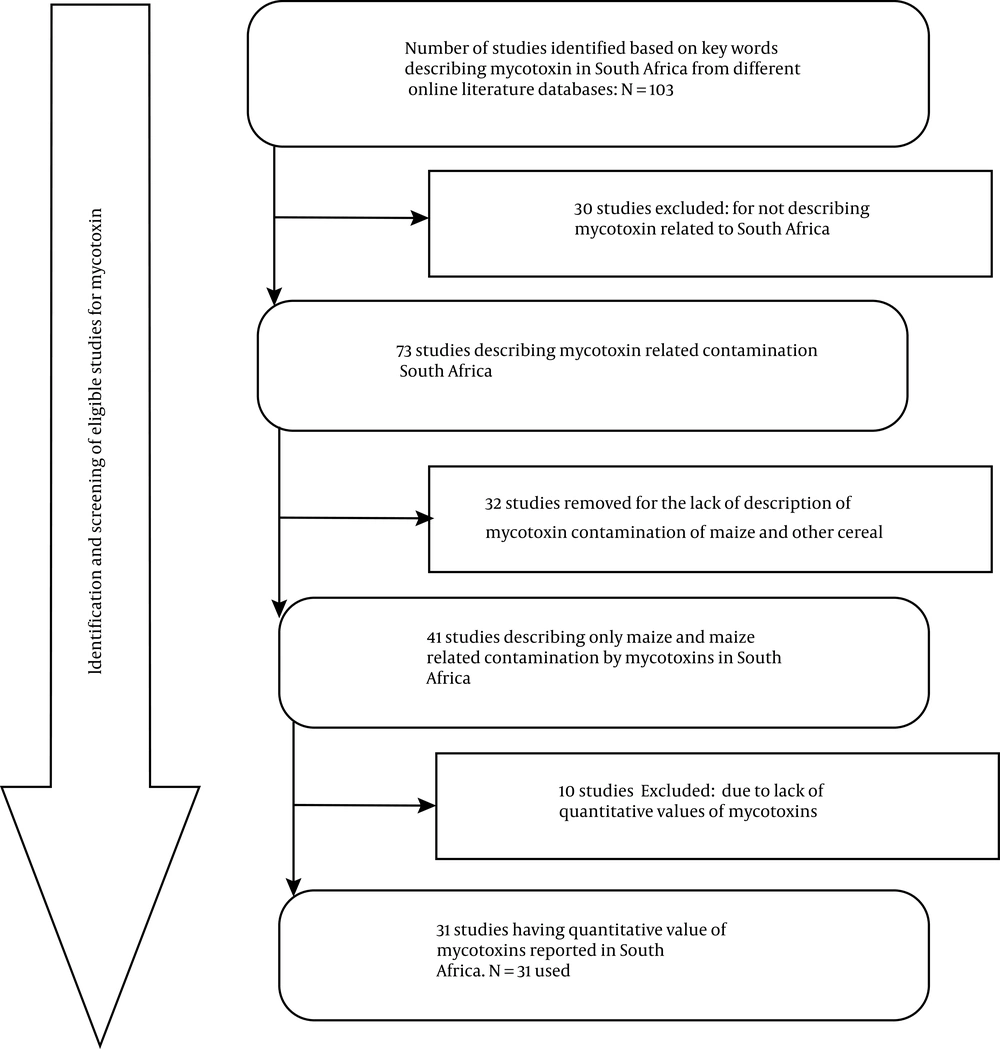
Scientific research articles indexed in PubMed, Google Scholar, Science Direct, and Web of Science were retrieved. Selected articles were all screened for duplication by two reviewers. The databases were compiled in a single spreadsheet and grouped according to provinces, books, book chapters, topics, abstracts, full articles, dissertations, thesis, and year of publication. Related topics were further grouped according to eligibility criteria and then analyzed for duplications, especially for conference publications and full articles. Risk bias (i.e. publication bias), as reported by Peters et al. (28), was not considered because of the limited number of studies describing mycotoxins contamination in maize and other cereals in South Africa. The search yielded 103 publications; however, these were further screened for eligibility criteria, resulting in a total of 73 mycotoxin-related publications relevant to this study (Figure 1). Since this scoping review was based on mycotoxin contamination of maize and other cereal food products in South Africa, related reports from other countries were excluded. In doing so, 32 of the considered publications were further excluded. Additional 10 publications were excluded due to the absence of data on mycotoxin quantification levels. Finally, only 31 publications/reports were analyzed in-depth for this scoping review (Figure 1).
4. Results and Discussion
This study describes the type of grains, particularly maize and products associated with mycotoxin contamination, the province where the cereals were obtained as well as the type and concentration of mycotoxins. Mycotoxins in maize and other cereals were confirmed in eight provinces (Table 1). These metabolites included deoxynivalenol (DON), nivalenol (NIV), FB, AF, ZEA, OTA, and patulin (PAT). The most common group of mycotoxins found across all investigated provinces was FB, followed by the AF. Maize only was confirmed to be affected by five (FB, AF, DON, OTA, ZEA) of the mycotoxin identified in South Africa. In a recent research on the South African perspective on the mycotoxin status of commercial maize and wheat crop annually produced, AFB1 was seldom present in commercial maize. In the same report, DON and FB were the most prevalent mycotoxins in all provinces that produce white and yellow maize (29).
| Province | Type of Mycotoxin | Food Stuff (s) | Mycotoxin Levels (µg/kg) | References |
|---|---|---|---|---|
| Eastern Cape | DON | Wheat | 3750 − 14360 | (30) |
| NIV | 320 − 1850 | |||
| EC (Bizana) | FB1 | Healthy corn | 0.2 - 0.55 | (31) |
| FB2 | 0.05 - 0.15 | |||
| EC (Centane) | FB1 | Healthy corn | 0.5 - 7.9 | |
| FB2 | 0.15 - 2.25 | |||
| Free State | FB1 | Corn meal | 475 | (32) |
| FB2 | 131 | |||
| Western Cape | FB1 | Corn grits | 190 | |
| FB2 | 120 | |||
| Eastern Cape | Total FBs | Good corn | 1985 | (33) |
| Low rate area | 700 | |||
| High rate area | 50 − 10150 | |||
| Moldy corn | ||||
| Low rate area | 600 − 25650 | |||
| High rate area | 4350 − 63200 | |||
| Good corn | 1989 | |||
| Low rate area | 4280 | |||
| High rate area | 6700 | |||
| Moldy corn | ||||
| Low rate area | 110 − 15040 | |||
| High rate area | 3770 − 140480 | |||
| Mpumalanga | FB1 | Maize | 630 | (34) |
| FB2 | 250 | |||
| Northern Cape | FB1 | 270 | ||
| FB2 | 170 | |||
| NS | FB1 and FB2 | Maize products (meal, braai pap and samp) | 0.1 − 250 | (35) |
| South Africa | AFs | Whole wheat and wheat products | 0.5 − 1.2 | (36) |
| DON | 500 − 18000 | |||
| ZEA | 4 | |||
| FBs | 1000 – 2000 | |||
| OTA | 1 − 4 | |||
| South Africa | AFs | Field wheat | 2 | |
| DON | 18000 | |||
| ZEA | 9 | |||
| FB1 and FB2 | 13000 | |||
| OTA | 2 | |||
| NS | AFB1 | Maltsters barley | 0.9 | (17) |
| DON | 832 | |||
| OTA | 0.9 | |||
| ZEA | 140 | |||
| NS | AFB1 | Maltsters malt | 1.3 | |
| DON | 362 | |||
| OTA | 1.3 | |||
| ZEA | 150 | |||
| Gauteng | DON | Retail barley | 150 | |
| ZEA | 170 | |||
| SA | DON | Wheat (2014 – 2018) | 202 – 397 (Mean conc.) | (29) |
| 361 – 593 (Max. conc.) | ||||
| White maize (2014 – 2017) | 110 – 1595 (Mean conc.) | |||
| Yellow maize (2014 – 2017) | 100 – 575 (Mean conc.) | |||
| FUM | White maize (2014 – 2017) | 24 – 2015 (Mean conc) | ||
| Yellow maize (2014 – 2017) | 57 – 1332 (Mean conc) | |||
| ZON | White maize (2014 – 2017) | 25 – 121 (Mean conc) | ||
| Yellow maize (2014 – 2017) | 23 – 113 (Mean conc) | |||
| KZN | FBs | Maize | 21.8 | (37) |
| AFs | 0.2 − 50 | |||
| Limpopo | FBs | 5.5 | ||
| AFs | 12 | |||
| Eastern Cape | FBs | 9 | ||
| AFs | 12 | |||
| Mpumalanga | FBs | 5.3 | ||
| AFs | 3.9 − 19 | |||
| NS | DON | Wheat flour | 29 | (22) |
| Maize | 294 | |||
| PAT | Apple juice | 210 a | ||
| KZN | AFM1 | Farm milk | 0.02 − 1.5 a | (24) |
| Retail milk | 0.01 − 3.1 a | |||
| KZN | FBs | Maize | 64 − 1035 | (38) |
| AFBs | 762 | |||
| OTA | 194135 | |||
| ZEA | ||||
| Eastern Cape | FB1 | Good maize | 2764 | (39) |
| FB2 | 1050 | |||
| DON | 4.7 | |||
| ZEA | 25 | |||
| FB1 | Moldy maize | 10.58 | ||
| FB2 | 14.14 | |||
| DON | 5.78 | |||
| ZEA | 135 | |||
| Mpumalanga, Limpopo & Gauteng | AFM1 | Milk (rural farm) | 0.15 | (40) |
| Milk (commercial farm) | 0.14 | |||
| Limpopo | AFB1 | Maize | 1 − 133 | (41) |
| FB1 | 12 − 8514 | |||
| Mpumalanga | AFB1 | < 1.0 | ||
| FB1 | 11 – 18924 | |||
| Limpopo | FB1 | Maize | 101 – 53863 | (42) |
| Porridge | 0.2 – 20 | |||
| Faeces | 0.3 – 464 | |||
| EC (Centane) | ||||
| (1997) | Total FB | Good home-grown maize | 575 | (43) |
| Moldy home-grown maize | 4845 | |||
| (2000) | Good home-grown maize | 975 | ||
| Moldy home-grown maize | 12905 | |||
| (2003) | Good home-grown maize | 2150 | ||
| Moldy home-grown maize | 43565 | |||
| KZN (Durban) | AFB | Commercial beers (Utshwala) | 20 - 400 a | (44) |
| KZN (Durban) | AFB | Home-brewed Beers (Isiqatha) | 12 a | (44) |
| KZN (Durban) | ZEA | Home-brewed beers (Isiqatha) | 2.6 - 426 a | (44) |
| KZN (Durban) | ZEA | Home-brewed beers(umqombothi) | 3 - 8 a | (44) |
| KZN (Durban) | OTA | Home-brewed beers (Isiqatha) | 1.5 – 2340 a | (44) |
| KZN (Durban) | OTA | Home-brewed beers(umqombothi) | 60 - 876 a | (44) |
| KZN (Durban) | OTA | Home-brewed beers (Imfulamfula) | 150 - 1100 a | (44) |
Mycotoxins in Maize, Maize-Based Food Products, And Other Cereals from Different Communities in South Africa
This scoping review highlights the importance of mycotoxin contamination and that it remains a serious concern for producers and consumers of maize and related cereal food products in the country. The high prevalence of mycotoxigenic fungi can be attributed to the limitations related to storage, particularly in urban areas. As shown in Table 1, maize is a major source of human dietary exposure to mycotoxins in South Africa, with implications in Africa, since the country is a major supplier to other countries on the African continent. Only a few studies have reported contamination of other cereals such as wheat (Triticum aestivum), barley (Hordeum vulgare), and sorghum (Sorghum bicolor) in this country, which can be attributed to these foods not commonly consumed in most parts of South Africa.
Maize is predominantly contaminated by Fusarium spp., especially F. graminearum, F. culmorum, F. verticillioides, F. proliferatum, and F. anthophilum. These are important fungal species that are the principal producers of mycotoxins, including FB, AF, and DON (38, 45). The high prevalence of mycotoxin production in provinces like Kwazulu-Natal, Gauteng, and Free State (Table 1) can be attributed to hot, dry, and humid climates. In this study, FBs (especially FB1) were the most common mycotoxin, followed by DON and AFs (Table 1). High FB levels was mostly present in home-grown maize.
This was confirmed in the Eastern Cape Province with levels as high as 1.4 × 108 µg/kg (33), followed by Limpopo province with FB levels of 101 – 53863 µg/kg (42). In South Africa, DON contaminates mainly wheat and wheat related products (36). The highest level of DON was recovered from wheat produced in the Eastern Cape Province, with 3750 - 14360 µg/kg (30), though a higher level (18000 µg/kg) was reported with the analyses of combined wheat and wheat-based products collected throughout South Africa (36). Nonetheless, DON toxicity under field conditions has not been reported in the country, although consumption of DON contaminated foods has been linked to outbreaks of human gastroenteritis in India (46) and food poisoning in China (47). DON is also prevalent in maize and home-brewed beer, where it was recovered at 294 and 2340 µg/kg, respectively, particularly in the rural areas of South Africa (22, 44). Limited information is available on AF contamination of different crops in South Africa. Available literature indicates that exposure to this toxin is restricted to maize consumption and few other crops affected by Fusarium species. It may also be directly consumed in other cereals, legumes, and related foodstuffs (31, 36) or indirectly with animal by-products, as summarized in Table 1. Other mycotoxins often encountered in South Africa include ZEA and OTA (17, 36, 38, 39, 44). OTA has been reported in South African traditional home-brewed beer at very high levels, ranging from 876 to 2340 µg/L, with the highest level reported in Kwazulu-Natal (31). This is a product that is consumed almost daily by the local population, and exposure to such high levels of OTA over prolonged period could elicit serious health complications with the human kidney. However, some studies conducted in South Africa have shown OTA levels in cereals within acceptable standards (17, 36, 48). ZEA levels up to 426 µg/L were identified in cereal-based products (44). These levels were noted to be above the maximum level of 100 µg/kg established by the European Commission (49) regulated in unprocessed cereals.
Although mycotoxin occurrences have been reported in cereals in South Africa, very few cases of human mycotoxicosis have been documented. Palanee (50) reported an association between exposure levels to FB1 and levels in patient's blood as well as brain lesions at Wentworth Hospital’s Neurosurgical Unit of Kwazulu-Natal province. Additionally, consumption of maize with high concentrations of FBs has been linked to increased risk of esophageal carcinoma in South Africa (33). In rural populations of the Eastern Cape Province, where maize and maize-based products are consumed in high volumes, FBs have been implicated in a high incidence of neural tube defects (51, 52). Recently, an association was reported between the AFB1 and cases of kwashiorkor, marasmus, and underweight in South Africa (2). With the increasing consumption of maize, particularly in rural subsistence farming communities in the country (5), where infrastructure is poor, there is bound to be an increase in mycotoxigenic fungi infections. Contributing factors such as changes in natural conditions (sporadic weather changes) and human activities (increased crop movement) have led to a potential increase in mycotoxin-related health problems.
There are a limited number of reports on the effect of mycotoxins on human and animal health in South Africa, which may be due to the low levels of research on the understanding of the impact of mycotoxins on food safety as well as their associated health consequences. To perform the current scoping review, we reviewed studies and reported published from 1980 to 2020. The findings highlight the economic importance of predominant mycotoxins in maize and other cereals like FBs, AFs, DON, and to a lesser extent, OTA and ZEA (36, 38, 53) in the country.
The methodological approach of the current study was not fully appraised due to the limited number of peer-reviewed articles describing mycotoxin contamination in maize and other cereals in South Africa. Sourcing data from non-academic institutions, particularly when such information was not available online, proved to be difficult. English was used as the search language in the study, as such also limiting the number of articles used for analysis
5. Conclusion
This study demonstrates high levels of contamination in maize and other cereal products by mycotoxins, notably FBs and AFs. These contaminations are sometimes associated with major health and economic challenges. There is, therefore, a need to increase efforts towards monitoring mycotoxins in food and feed as well as adopting regulatory measures that are effective, sustainable, and scalable in reducing and associated health and economic burden. It is important to empower farmers and other stakeholders to improve on good hygiene and farming practices, particularly at farm and storage levels. Such interventions can limit mycotoxin-associated food contamination, which in turn will translate into declined exposure to these toxic secondary metabolites among humans and animals. This is particularly important as other mycotoxins, especially the emerging ones, can be considered in the regulation of food trade. With changes in global climate scenarios, there may be an increased mycotoxin production, and different or emerging mycotoxins may become important (53-55).