1. Background
Coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 infection emerged in Wuhan, China, in December 2019 (1). This disease has now spread to all the regions and countries of the world, including Iran (2). Today (October 2, 2020), the virus has infected more than 461,044 individuals causing more than 26,380 deaths in Iran.
The transmission of COVID-19 occurs via close contact and most often through talking, coughing, and sneezing (3). Fever, cough, fatigue, shortness of breath, and loss of smell and taste are the common main symptoms of the disease. The range of COVID-19 manifestations varies from asymptomatic infection to acute respiratory infection (4-6). For instance, previous studies have suggested that approximately 81% of patients with novel coronavirus infection are mild cases, 14% of the cases develop the severe disease requiring hospitalization and oxygen therapy, and 5% of the cases require admission to an intensive care unit (ICU) (5, 7). Previous studies have also shown that disease severity is associated with increased age, diabetes, immunosuppression, organ failure, white blood cell count, neutrophil count, lymphocyte count, D-dimer, albumin, creatinine count, and procalcitonin level (4, 7-10).
In the fight against COVID-19, the clinical and laboratory predictors of progression toward severe and fatal forms need to be recognized. The recognition of these risk factors can help to classify risks, optimally allocate limited human and technical resources, and determine prevention strategies, and target high-risk populations using potential therapeutics. Additionally, the identification of laboratory parameters associated with the severity of the disease or mortality can help to predict the prognosis of the disease and take active treatment measures as soon as possible.
2. Objectives
This study aimed to compare the laboratory parameters between patients with severe and non-severe COVID-19 in an Iranian population.
3. Methods
Simultaneously with the report of the first case of COVID-19, a comprehensive electronic system was created for gathering all the data related to the confirmed and suspected COVID-19 patients in Shahroud, northeast of Iran. The general and specific data of patients, such as age, gender, height, weight, residence place, sampling method, comorbidities, smoking history, medicinal use history, medical history, cardiac monitoring, oxygen therapy, radiological assessment (e.g., chest X-ray or computed tomography scan), and laboratory parameters were recorded in this system. The general condition of the patients, real-time reverse transcription-polymerase chain reaction (RT-PCR) results, laboratory parameters, and outcome data were obtained from this electronic system.
In this study, a confirmed case of COVID-19 was defined as someone whose RT-PCR was positive based on nasal or nasopharyngeal swabbing. The severity of the disease was divided into mild, intensive, and critical types. Mild type patients were defined as admitted patients with mild clinical manifestations without unusual imaging data. The intensive type was defined as patients with respiratory distress requiring ICU admission but without the need for mechanical ventilation or shock. Critical type patients were those patients admitted to the ICU with respiratory failure with or without other organ failure or patients requiring mechanical ventilation. Finally, the patients were divided into two groups based on the severity of the disease. The patients with the intensive and critical types of the disease were assigned to the severe group, and those with the mild type were assigned to the non-severe group.
The chi-square test was used to compare categorical variables. For continuous variables, means were compared using the independent samples t-test or Mann-Whitney U test based on the result of the normality test. The variables with a p-value of less than 0.2 in univariate analysis were included in multivariate analyses. Multivariate analyses of prognostic factors were performed using logistic regression. The area under the curve (AUC), cut-off value, sensitivity, and specificity were estimated for each of the significant predictive factors by the receiver operating characteristic (ROC) curve. In ROC analysis, disease severity was used as a reference variable. Youden’s index was used to determine the optimal cutting point. A P-value of less than 0.05 was considered statistically significant.
4. Results
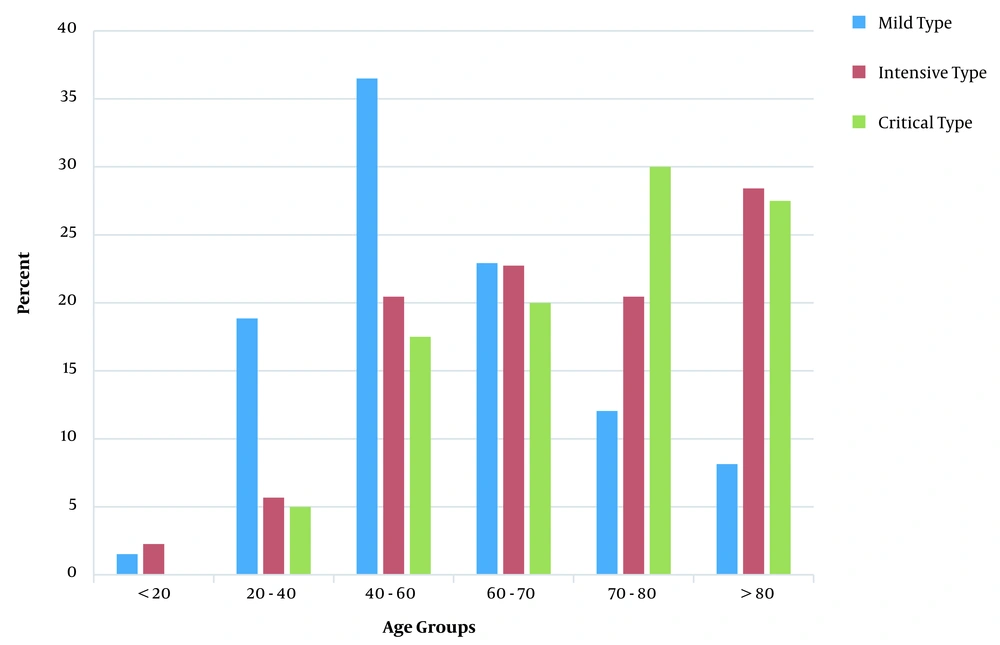
A total of 731 patients [407 male cases (55.56%)] participated in this study. The mean (median) age value and age range of the patients were 58.37 (59.80) and 1 - 98 years, respectively. The intensive and critical types of COVID-19 were more frequently observed in individuals over 60 and under 20 years of age (Figure 1). The univariate analysis showed that erythrocyte sedimentation rate, international normalized ratio (INR) for prothrombin time, lactate dehydrogenase (LDH), neutrophil count, lymphocyte count, hemoglobin, serum glutamic-oxaloacetic transaminase (SGOT), serum glutamic-pyruvic transaminase (SGPT), alkaline phosphatase, SGOT/SGPT ratio, serum urea, creatinine, and neutrophil/lymphocyte ratio (NLR) had a statistically significant association with disease severity (P < 0.05; Table 1).
| Variables | Non-severe Type | Sever Type | P-Value |
|---|---|---|---|
| Gender (male) | 327 (54.59); 272 (45.41) | 80 (60.61); 52 (39.39) | 0.208 |
| Age (y) | 56.20 ± 16.69 | 68.37 ± 16.93 | < 0.001 |
| Body mass index | 0.015 | ||
| < 25 | 172 (28.81) | 54 (41.54) | |
| 25 - 29.99 | 277 (46.40) | 52 (40.00) | |
| ≥ 30 | 148 (24.79) | 24 (18.46) | |
| Erythrocyte sedimentation rate | 34.86 ± 22.11 | 44.31 ± 30.85 | < 0.001 |
| International normalized ratio | 1.14 ± 0.24 | 1.37 ± 0.84 | < 0.001 |
| Serum potassium | 4.08 ± 0.49 | 4.18 ± 0.59 | 0.034 |
| Serum sodium | 135.96 ± 3.73 | 135.079 ± 7.79 | 0.697 |
| Lactate dehydrogenase | 466.33 ± 191.51 | 666.98 ± 290.98 | < 0.001 |
| Neutrophil ratio (%) | 67.37 ± 12.64 | 74.52 ± 13.86 | < 0.001 |
| Lymphocyte ratio (%) | 27.44 ± 12.27 | 20.34 ± 13.11 | < 0.001 |
| Platelet count (105/mm3) | 2.06 ± 0.69 | 2.09 ± 0.94 | 0.611 |
| Monocyte ratio (%) | 2.88 ± 1.22 | 2.94 ± 1.46 | 0.633 |
| Eosinophil ratio (%) | 2.39 ± 0.96 | 2.35 ± 1.09 | 0.709 |
| Hemoglobin (g/dL) | 13.41 ± 1.87 | 12.82 ± 2.44 | 0.002 |
| Serum glutamic-oxaloacetic transaminase | 35.60 ± 28.34 | 51.40 ± 56.41 | < 0.001 b |
| Serum glutamic-pyruvic transaminase | 34.10 ± 31.41 | 41.97 ± 77.31 | 0.059 b |
| Alkaline Phosphatase (IU/L) | 189.39 ± 80.85 | 220.98 ± 104.82 | < 0.001 |
| Serum glutamic-oxaloacetic transaminase/serum glutamic-pyruvic transaminase ratio | 1.25 ± 0.64 | 1.64 ± 0.94 | < 0.001 |
| Serum urea | 34.27 ± 19.57 | 50.67 ± 34.43 | < 0.001 |
| Serum creatinine | 1.06 ± 0.65 | 1.22 ± 0.71 | 0.008 |
| Neutrophil/lymphocyte ratio | 3.58 ± 3.43 | 5.94 ± 5.50 | < 0.001 b |
a Values are expressed as No. (%) or mean ± SD.
b Mann-Whitney U test.
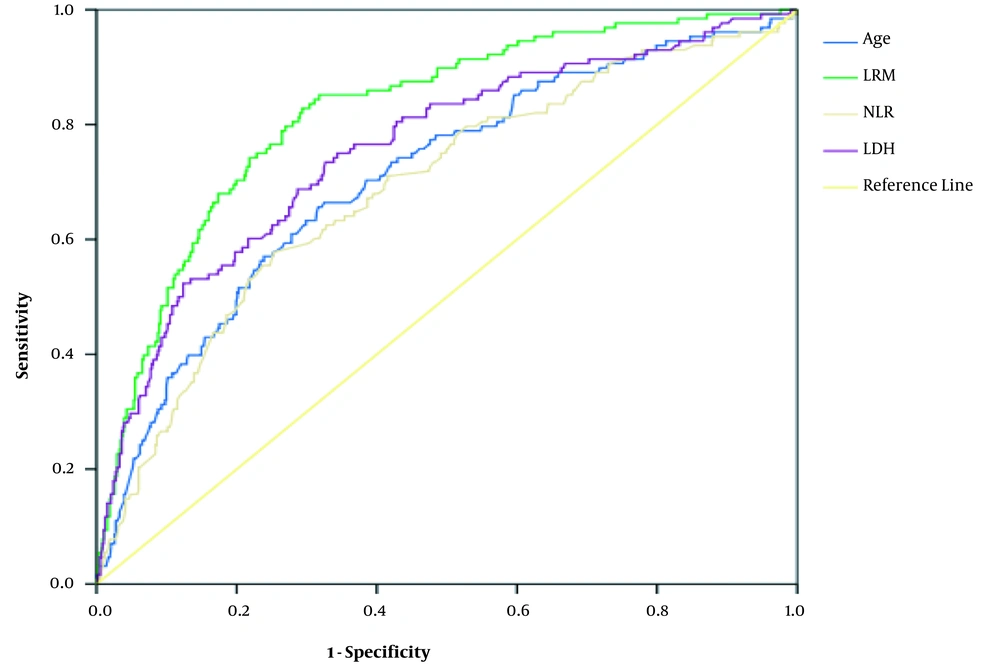
The patients’ age (OR = 1.03, 95% CI: 1.02 - 1.05), INR (OR = 2.09, 95% CI: 1.11 - 3.96), LDH (OR = 1.003, 95% CI: 1.001 - 1.1.003), and NLR (OR = 1.08, 95% CI: 1.02 - 1.14) were associated with the severity of COVID-19 in the multivariate logistic regression (Table 2). The AUC of LDH, NLR, INR, and logistic regression for the diagnosis of disease severity ranged from 0.62 for INR to 0.82 for logistic regression. The details of the AUC and optimal thresholds of each independent factor are shown in Table 3 and Figure 2.
| Independent Variables | Odds Ratio | 95% Confidence Interval |
|---|---|---|
| Age (y) | 1.03 | 1.02 - 1.05 |
| International normalized ratio | 2.07 | 1.11 - 3.87 |
| Lactate dehydrogenase | 1.003 | 1.001 - 1.003 |
| Neutrophil/lymphocyte ratio | 1.08 | 1.02 - 1.14 |
| Variables | Area Under the Curve (95% CI) | P-Value | Optimal Threshold | Sensitivity | Specificity | Youden’s Index |
|---|---|---|---|---|---|---|
| Age | 0.71 (0.66 - 0.76) | < 0.001 | ≥ 65 | 0.656 | 0.677 | 0.334 |
| Lactate dehydrogenase | 0.76 (0.71 - 0.81) | < 0.001 | ≥ 487 | 0.742 | 0.673 | 0.415 |
| Neutrophil/lymphocyte ratio | 0.69 (0.64 - 0.74) | < 0.001 | ≥ 3.85 | 0.568 | 0.745 | 0.313 |
| International normalized ratio | 0.62 (0.56 - 0.67) | < 0.001 | ≥ 1.28 | 0.432 | 0.813 | 0.245 |
| Logistic regression model | 0.82 (0.78 - 0.86) | < 0.001 | ≥ 0.151 | 0.828 | 0.706 | 0.534 |
5. Discussion
This cross-sectional study revealed that age, INR, NLR, and LDH were associated with COVID-19 severity. Although different definitions of COVID-19 disease severity in different studies make the comparison partly difficult, the observed association between age and disease severity is consistent with the results reported from other studies (7, 11-13). The aging process causes anatomical changes in the lung and other supportive extra pulmonary structures, including the chest, spine, and respiratory muscles (14). These changes in the anatomical structure of the lung lead to an unfavorable respiratory mechanism and reduced gas exchange. Therefore, due to lung involvement in COVID-19, the severity of COVID-19 is expected to increase with age.
It should also be noted that the humoral and cellular immune function of the human body decreases with age (15). The age-dependent defects in the immune function may reduce the human body’s resistance to COVID-19 and increase the severity of the disease. However, since the risk of chronic diseases increases with age, an increase in the severity of COVID-19 in the elderly may be associated with comorbidities that should be considered.
According to the findings of the current study, the NLR was another factor with a direct association with the severity of COVID-19. The results of the present study are consistent with the results reported from several other studies in this regard (16-18). For instance, in a study performed by Liu et al. (16), the NLR was reported as an independent risk factor for COVID-19. Moreover, according to the findings of Qin et al. (17), the number of neutrophils was higher than the number of lymphocytes in severe cases of COVID-19 than in non-severe cases.
Moreover, Mo et al. (18) reported that severe cases had a higher level of neutrophils in comparison to general patients. Therefore, it can be concluded that the NLR tends to be high in severe cases of COVID-19. Increasing the number of neutrophils in the body releases reactive oxygen species, which damages familiar and foreign cells and reduces the number of lymphocytes needed to fight infectious diseases (19); therefore, a higher NLR means higher inflammation and consequently more damage.
Similar to the findings of the present study, the association of elevated LDH values with the poor prognosis of COVID-19 has been reported in other disease, including cancer and infection (9, 13, 20-22). The LDH is observed as an intracellular enzyme in all the cells of the human body (23). The abnormal values of LDH can be caused by damage to various organs and reduced oxygen supply (21). The infection with COVID-19 may damage cytokine-mediated tissue and increase the release of LDH (24). Because LDH is present in the lung tissue, it can be expected to have an increase in LDH release in patients with severe COVID-19.
In this study, LDH was observed as the best variable to discriminate between non-severe and severe COVID-19 cases. Based on the thresholds obtained from the ROC curve, the optimal LDH threshold for the discrimination of COVID-19 cases was 487. Accordingly, the progression of the disease from LDH of ≥ 487 changed from non-severe to severe. Therefore, clinicians should pay further attention to patients with LDH of > 487. It should also be noted that other variables, including age, NLR, and INR, can help to discriminate between non-severe and severe COVID-19 cases (Figure 2). As a result, clinicians can use these variables to improve therapeutic effects and reduce disease severity.
This study was conducted with several limitations. Firstly, there was no possibility to study all the laboratory factors in this study; therefore, adding other factors may change the sensitivity and specificity values. Secondly, missing data in some variables might have reduced the representativeness of the samples and generalizability of the findings. However, as there were no missing data in the studied variables, it does not seem to have a significant impact on the findings of the current study. Finally, it should be noted that this study was conducted using an observational design; consequently, the observed association between the laboratory findings and disease severity cannot be considered a causal association. Therefore, it is recommended to further test these factors in longitudinal studies.
5.1. Conclusion
The results of this study revealed that LDH, NLR, and INR could help to discriminate between non-severe and severe COVID-19 cases, among which LDH is the best predictor of severe COVID-19. In addition, the combination of three clinical indicators can further predict severe COVID-19. Given that there is currently no effective treatment for COVID-19, physicians can diagnose severe cases of COVID-19 in the early phase using these clinical indicators and take active treatment measures as soon as possible to reduce the case fatality rate.