1. Background
COVID-19 pandemic, which started in late 2019, has brought various ups and downs all over the world (1). Regarding the vaccine shortage as well as the absence of effective treatment in many countries, including Iran, actions intended to restrict contacts and forcing to isolation are widely accepted as main policies for controlling this extremely contagious disease (2, 3). These actions, in combination with a community commitment to respect public health protocols, were highly useful during the first wave of the pandemic; as a result, after two months, the situation almost came under control, and the number of cases and hospitalization rates were decreased significantly. Unfortunately, due to relaxed restrictions that resulted in declined distancing (for instance, through holding ceremonies) and several other reasons, Iran soon faced the second wave of the COVID-19 pandemic (4). Scientists believe that evaluating clinical features, disease severity, outcomes, and other features during an epidemic can help health policymakers to make better decisions (5). The main presentations, imaging findings, vulnerable groups, and etc. may change during such a high contagious viral epidemic that has no cure yet.
2. Objectives
In this line, the current study aimed to compare patients’ features in the first two waves of the COVID-19 pandemic in the city of Tehran, Iran. Considering the findings, Iranian health policymakers may provide more helpful information for both health staff and the general population about symptoms that may require more evaluation as a suspect case of COVID-19.
3. Methods
3.1. Study Design
The current cross-sectional study intended to investigate and compare the epidemiological differences of patients in the first and second waves of the COVID-19 pandemic in terms of demographic and baseline characteristics, presenting symptoms, including primary vital signs, imaging findings, and outcome. This study was performed using information available in the databank of the Tehran Emergency Medical Services (EMS) Center and the Medical Care Monitoring Center (MCMC) of Iran’s Ministry of Health and Medical Education (MoHME).
The research purpose and methodology were subjected to scrutiny by the Ethics Committee of the Tehran University of Medical Sciences (code: IR.TUMS.MEDICINE.REC.1399.854), Tehran EMS center, and MoHME. All information were analyzed anonymously and in accordance with the principles of confidentiality.
3.2. Study Population
All suspected/confirmed COVID-19 cases, based on the World Health Organization (WHO) definitions updated on December 16, 2020 (6), who were transferred by ambulance to hospitals located in the city of Tehran from 2-20-2020 to 8-5-2020 were considered eligible. No exclusion criterion was considered in this study. The sample size was calculated for each variable, which was estimated less than 1000 in all circumstances; however, data of several COVID-19 cases were available in our database, and with the advice of our consultant methodologist, 5000 cases were randomly selected in this study, which was equal to almost half of all recorded cases in the database.
3.3. Data Gathering
The first wave of the COVID-19 epidemic was from February 20 to May 04, 2020, and the second wave was from May 05 to August 05, 2020. Data for both waves were collected using a researcher-made checklist. By reviewing the pre-hospital and hospital records of patients, information on the following variables were recorded for all cases: age, gender, behavioral risk factors including cigarette smoking and opium abuse, underlying diseases, primary symptoms, history of recent contact with a COVID-19 patient, O2 saturation measured by the EMS technician, positive findings in lung computed tomography (CT) scan, need for intubation during the hospital stay, the hospitalized ward, length of hospitalization, and death, if occurred.
3.4. Statistical Analysis
We analyzed all available data for each variable. Cases with missing data were excluded. The Chi-Square test was used to investigate the association between qualitative and quantitative variables, and if defaults were not met, the fisher’s exact test was applied. The independent t-test was used to compare the mean value of quantitative variables in the two waves of the pandemic. Also, we used multivariable logistic regression to assess the main study outcomes in suspected/confirmed COVID-19 patients in the second wave compared to the first one. Data analysis was administered using Stata software version 14. Statistical significance was considered when P-value < 0.05.
4. Results
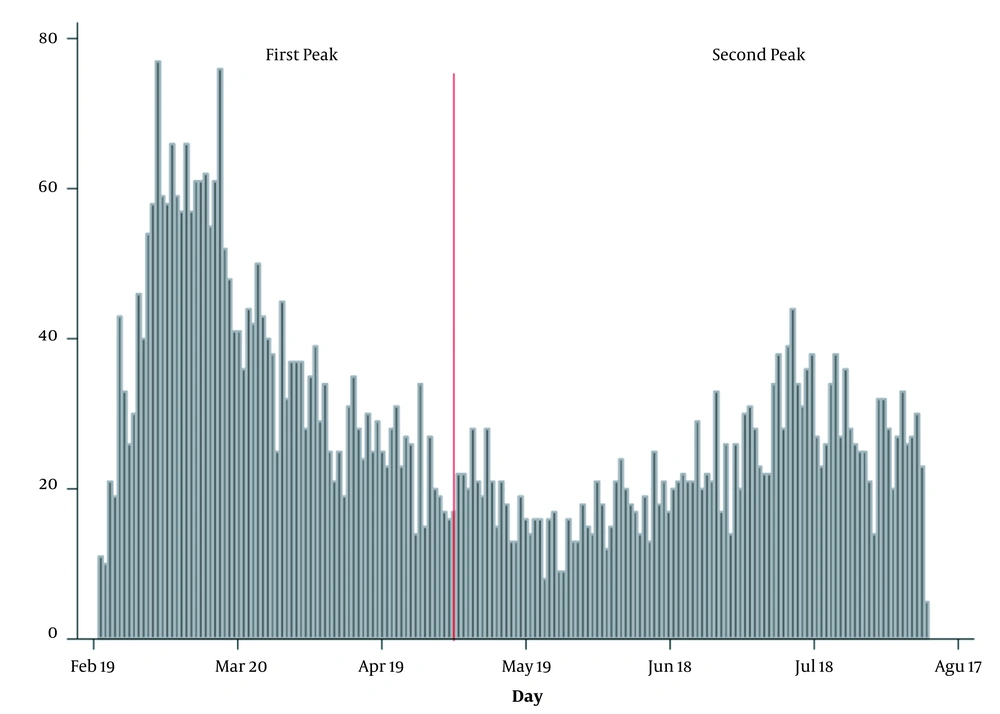
In this study, data of 5000 suspected/confirmed COVID-19 cases were analyzed (2773 cases belonged to the first wave and 2227 to the second one) (Figure 1). In the following, the findings are presented. The mean age of all patients was 58.8 years (SD = 19.8), while for cases identified during the first and second waves it was 57.1 (SD = 19.8) and 61/0 (SD = 19.6) years, respectively. The mean age of cases identified during the first wave was significantly lower compared to the second wave (P < 0.001).
Totally, 2919 cases (58.8%) were male, and this percent for the first and second wave was 59.4% and 57.2%, respectively. The gender ratio difference was not statistically significant between the two waves (P = 0.117). Almost 2.3% of all studied patients had a history of smoking, and 2.5% had a history of opium abuse (addiction). The prevalence of these factors in the second wave was more than twice of the first wave, and the observed difference was statistically significant (p < 0.05) (Table 1).
| Variable | First Wave (N = 2773), No. (95% CI for %) | Second Wave (N = 2227), No. (95% CI for %) | P-Value |
|---|---|---|---|
| Gender | 0.117 | ||
| Male | 1646 (57.5 - 61.2) | 1273 (55.1 - 59.2) | |
| Female | 1127 (38.8 - 42.5) | 954 (40.8 - 44.9) | |
| Behavioral risk factors | |||
| Cigarette smoking | 33 (0.95 - 1.93) | 73 (2.5 - 4.0) | < 0.001 |
| Opium abuse | 42 (1.3 - 2.4) | 71 (2.5 - 3.9) | 0.004 |
| Underline disease | |||
| Cancer | 53 (1.4 - 2.4) | 49 (1.6 - 2.8) | 0.472 |
| Hepatic failure | 12 (1.9 - 6.8) | 9 (1.4 - 6.7) | 0.876 |
| Diabetes mellitus | 226 (7.1 - 9.2) | 249 (9.9 - 12.5) | < 0.001 |
| Hypertension | 102 (3.6 - 5.3) | 273 (10.9 - 13.6) | < 0.001 |
| Chronic hematologic disease | 7 (0.07 - 0.44) | 14 (0.30 - 0.96) | 0.041 |
| HIV/AIDS | 1 (0.0 - 0.11) | 3 (0.0 - 0.29) | 0.330 |
| Immune deficiency | 10 (0.14 - 0.58) | 6 (0.05 - 0.48) | 0.570 |
| Cardiovascular disease | 258 (8.2 - 10.4) | 212 (8.3 - 10.7) | 0.795 |
| Renal failure | 64 (1.7 - 2.9) | 44 (1.4 - 2.5) | 0.422 |
| Asthma | 43 (1.1 - 2.0) | 28 (0.79 - 1.7) | 0.384 |
| Chronic obstructive pulmonary disease | 42 (1.1 - 2.0) | 50 (1.6 - 2.9) | 0.056 |
| Cerebrovascular disease | 37 (0.91 - 1.8) | 27 (0.76 - 1.7) | 0.703 |
| Others | 120 (3.6 - 5.1) | 85 (3.0 - 4.6) | 0.365 |
| Primary symptoms | |||
| Fever | 945 (32.3 - 35.8) | 689 (29.0 - 32.9) | 0.019 |
| Cough | 1041 (35.7 - 39.3) | 732 (30.9 - 34.8) | < 0.001 |
| Myalgia | 454 (15.0 - 17.7) | 563 (23.5 - 27.1) | < 0.001 |
| Shortness of breath | 1284 (44.4 - 48.2) | 1273 (55.1 - 59.2) | < 0.001 |
| Loss of consciousness | 279 (8.9 - 11.2) | 396 (16.2 - 19.4) | < 0.001 |
| Loss of sense of smell | 10 (0.17 - 0.71) | 24 (0.65 - 1.5) | 0.013 |
| Loss of sense of taste | 4 (0.0 - 0.37) | 17 (0.40 - 1.1) | 0.007 |
| Seizure | 9 (0.15 - 0.70) | 13 (0.27 - 0.90) | 0.467 |
| Headache | 6 (0.11 - 0.99) | 105 (3.8 - 5.6) | < 0.001 |
| Vertigo | 3 (0.0 - 0.57) | 47 (1.5 - 2.7) | < 0.001 |
| Limb paresthesia | 1 (0.0 - 0.26) | 16 (0.37 - 1.1) | 0.016 |
| Limb plegia | 2 (0.0 - 0.43) | 5 (0.03 - 0.42) | 1.0 |
| Chest pain | 10 (0.34 - 1.4) | 88 (3.1 - 4.8) | < 0.001 |
| Abdominal pain | 9 (0.22 - 1.0) | 51 (1.7 - 2.9) | < 0.001 |
| Nausea | 19 (0.74 - 1.9) | 112 (4.1 - 5.9) | < 0.001 |
| Vomiting | 14 (0.47 - 1.5) | 80 (2.8 - 4.4) | < 0.001 |
| Diarrhea | 12 (0.37 - 1.3) | 93 (3.3 - 5.0) | < 0.001 |
| Anorexia | 6 (0.08 - 0.76) | 141 (5.3 - 7.3) | < 0.001 |
| History of recent contact with COVID-19 patient | 662 (22.3 - 25.5) | 682 (28.7 - 32.5) | < 0.001 |
| O2 saturation < 93% | 1227 (42.4 - 46.1) | 750 (31.7 - 35.6) | < 0.001 |
| Positive findings in lung CT scan | 891 (80.9 - 85.4) | 1460 (93.4 - 95.7) | < 0.001 |
| Intubation during the hospital stay | 551 (18.4 - 21.4) | 366 (14.9 - 18.0) | 0.002 |
| Ward of hospitalization | 0.117 | ||
| General | 1039 (35.7 - 39.3) | 855 (36.4 - 40.4) | |
| Isolation | 883 (30.1 - 33.6) | 650 (27.3 - 31.1) | |
| Intensive Care Unit | 851 (29.0 - 32.4) | 722 (30.5 - 34.4) | |
| Occurring death consequence | 560 (18.7 - 21.7) | 400 (16.4 - 19.6) | 0.046 |
Abbreviation: CI, confidence interval.
aMeasured by EMS technician at the scene;
Concerning the history of other diseases, the acquired immunodeficiency syndrome (AIDS) with a prevalence of 0.1% was the least prevalent co-existing disease, while diabetes mellitus (DM) was the the most frequent one (9.5% prevalence). In addition, there was a statistically significant difference between the two investigated waves concerning DM, chronic hematologic diseases, and hypertension (HTN); and generally, the history of having these 3 diseases was significantly higher in the second wave’s patients than that of the first one (P < 0.05). Also, the prevalence of chronic pulmonary diseases, except for asthma, was more common in the second wave than the first one (2.2% and 1.5, respectively; P = 0.056). There was no statistically significant difference between the investigated waves concerning other diseases (P > 0.05) (Table 1).
Concerning primary symptoms of all studied patients, the most common primary symptom was shortness of breath (51.1%), followed by cough (35.5%) and fever (32.7%). On the other hand, loss or decreased sense of smell, loss or decreased sense of taste, seizure, extremities’ paresthesia, and plegia were the least common (less than 1%) primary symptoms. Skin lesion was not reported as a primary symptom in any cases during the first wave. The prevalence of fever, as a primary symptom, was decreased from 34.1% in the first wave to 30.9% in the second wave (P = 0.019), and the prevalence of cough was decreased from 37.5% in the first wave to 32.9% in the second wave (P < 0.001). Except for these two symptoms, the prevalence of all other primary symptoms in the second wave was more than that of the first one, and these increases had statistically significant differences (except for the seizure and extremities’ plegia) (P < 0.05) (Table 1).
In terms of recent contact with a COVID-19 patient, those in the first case reported lower contacts compared to those in the second wave, which was statistically significant (P < 0.001) (Table 1). Of all studied patients, 39.5% had a low O2-saturation level (less than 93%) when examined by an emergency medical technician. However, the prevalence of hypoxic patients was 44.2% in the first wave and decreased to 33.7% in the second wave (P < 0.001) (Table 1).
Of all studied patients who underwent lung CT scan, 89.9% had positive findings related to the diagnosis of COVID-19, which the prevalence of such findings was significantly higher for patients in the second wave (94.6% vs 83.2, respectively; P < 0.001) (Table 1). In addition, 18.3% of all patients received intubation, which was significantly higher during the first wave as compared to the second (19.9 vs 16.4, respectively; P = 0.002) (Table 1). Regarding the hospitalized ward, 37.9% of patients were admitted in the general ward, 30.7% in the isolation ward, and 31.5% in the Intensive Care Unit (ICU). There was no statistically significant difference between the first and second waves concerning the hospitalized ward (P = 0.117) (Table 1).
Overall, the mean length of hospitalization was 5.5 (SD = 7.7) days. On average, the length of hospitalization was longer in the first wave than that of the second wave (P = 0.008). Eventually, 960 patients (19.2%) of all studied cases ended with death. The mortality rate was significantly higher in the first wave than that of the second wave (20.2% and 18%, respectively) (Table 1).
According to the results of the logistic regression, the frequency of positive lung CT-scan findings was higher in the second way by 3.4-times (95% CI: 2.51 to 4.55) than that of the first wave (P < 0.001). Although the odds of O2 saturation < 93% and intubation during hospitalization in the second wave were 1.53 and 1.95-times lower than that of the first wave, respectively. Meanwhile, there was no statistically significant difference between the two waves concerning the odd ratio of death (P = 0.212) (Table 2).
| Variable | OR for Second Wave | 95% CI for OR | P-Value |
|---|---|---|---|
| Ward of hospitalization | |||
| General | Reference | - | - |
| Isolation | 1.07 | 0.868 to 1.33 | 0.510 |
| Intensive Care Unit | 0.784 | 0.636 to 0.966 | 0.022 |
| Positive findings in lung CT Scan | 3.38 | 2.51 to 4.55 | < 0.001 |
| O2 saturation < 93% | 0.653 | 0.543 to 0.786 | < 0.001 |
| Intubation during the hospital stay | 0.512 | 0.403 to 0.650 | < 0.001 |
| length of hospital stay > 5 day | 1.18 | 0.986 to 1.41 | 0.071 |
| Occurring death consequence | 1.17 | 0.916 to 1.48 | 0.212 |
Abbreviation: OR, odds ratio.
5. Discussion
The analyses performed on the features of suspected/confirmed COVID-19 patients in the present study showed that, except for the gender ratio and the ward of hospitalization, there were interesting differences between the two waves of the COVID-19 pandemic. Based on our findings, those infected during the second wave were older than those in the first wave, which the observed difference was statistically significant. However, in a similar study conducted in the city of Babol (located in Mazandaran province in the north of Iran), Jalali et al. (7) reported that the mean age of affected cases was significantly lower during the second wave compared to the first wave of the COVID-19 pandemic. In contrast to the study by Jalali et al. (7) and the present study, Soriano et al. (8) found no significant changes in this regard and reported no significant mean age difference between the COVID-19 patients’ of the first and second waves in Madrid, Spain. It seems that the evidence regarding the variable of age is still inconclusive. Those with a history of cigarette smoking or opium abuse were significantly more prevalent in the second wave compared to the first one. DM and HTN were more common in patients of the second wave. In line with this, Jalali et al. (7), in a study carried out in Iran, reported that comorbidities were more prevalent in the second wave compared to the first one. Age, social habits, and underlying disease, especially HTN, are considered risk factors for COVID-19 infection (9-12). Hence, it can be argued that the elderly, smokers, addicts, and people with underlying diseases such as HTN are at increased risk of COVID-19 infection. In addition, one can interfere that the reduction of compliance with health protocols pose an extra risk to these groups.
In contrast to our findings, Jalali et al. (7) reported that while in the first wave, men were more affected by the COVID-19 infection, during the second wave, it became reversed (i.e., women were more affected). In our study, the men had a higher proportion of the affected cases in both waves. In contrast to our study and also Jalali et al. (7) study, which both were conducted in Iran, Soriano et al. reported the women preference in both first and second waves of the COVID-19 pandemic in Madrid, Spain (8). Hence, evidence about gender preference in various waves of the COVID-19 are inconclusive.
Concerning clinical symptoms, fever and cough, which were the most common symptoms reported by the patients in the first wave, were significantly decreased in the second wave. On the other hand, symptoms such as myalgia, headache, dizziness, and gastrointestinal symptoms were reported more frequently by the patients in the second wave. Such alterations in clinical presentation and occurrence of non-respiratory symptoms are also mentioned by Jalali et al. (7), who also assessed epidemiologic aspects of the first and second waves of COVID-19 pandemic in the city of Babol. At the early onset of the COVID-19 outbreak, there was an extra emphasis on fever, cough, and dyspnea presentations; so that these three symptoms had been introduced as the main symptoms of COVID-19 in the society in different ways; but when more evidence became available more symptoms were identified and introduced (13, 14), which were shared rapidly and became available to the public. Therefore, concurrent with the health staff, the public’s knowledge about this disease also has been raised, which resulted in paying more attention to possible presentations. This issue may be the main reason for the increased report of non-respiratory symptoms by patients.
The report of probable recent contact with COVID-19 patients in the second wave was more than that of the first wave. This finding was conceivable after the further prevalence of the disease in the community and also because of passing more time from the beginning of the outbreak. Hence, an increased number of infected cases is associated with enhanced risk of contact of a healthy individual with infected cases. In other words, this is a defective cycle in which the control of disease depends on its break, and restricting actions intend to intervene in the transmission cycle (15-17).
In the present study, we also investigated the frequency of patients with O2sat < 93%, which was recorded by the EMS technician. According to the findings, the frequency of patients with O2sat < 93% was higher in the first wave compared to the second wave. Also, the frequency of patients who received intubation in the hospital in the first wave was more than that of the second wave. Cases with positive findings in lung CT-scan were more prevalent in the second wave than that of the first wave. Also, hospitalization duration was longer in the first wave than the second one. Eventually, mortality rate was higher in the first wave than the second wave.
Comparison of findings related to the abovementioned five variables revealed an important issue that patients transferred to the hospital by ambulance during the first wave had worse health conditions than those transferred during the second wave. Soriano et al. (8) also reported that "the proportion of patients who experienced severe clinical symptoms was significantly lower during the second wave of COVID-19 pandemic in Madrid, Spain". Similar results are presented by Elshazli et al., who performed a meta-analysis on published papers in which the first and second waves were compared in terms of COVID-19 patients’ characteristics, mainly their gastroenterology manifestations. They reported that patients in the first wave, to some extent, had a higher risk of being hospitalized, ventilated, ICU admitted, and expired. In other words, their analysis revealed worldwide improvement of COVID-19 patients’ outcomes during the second wave compared to the first one (18).
We believe that one of the reasons justifying this finding could be the improvement of hospitals’ capacity for admitting COVID-19 patients and also the increase of people’s awareness to call the EMS sooner. However, the decrease in mortality rate and the mean period of hospitalization can be attributed to the improvements in therapeutic procedures, too.
5.1. Limitations
Although in this epidemiologic study, we tried to consider important variables as much as possible, there are several other variables that could be considered. One of the main limitations of the present study is the lack of patients’ categorization based on disease severity. Also, patients’ outcome depends on applied therapeutic protocols, such as intubation, the mean period of hospitalization, and the final outcome, that were not considered in the present study. Another noticeable point is that the number of COVID-19 symptoms has increased over time; hence, they might not be reported by the patients or were not registered by the technicians. There may be some confounding variables that were not considered in the methodology of the present study. For example, the “period of hospitalization” could be affected by the hospitals’ bed occupying rate and also admission protocols that have changed between the two waves.
5.2. Conclusions
The current study investigated the features of suspected/confirmed COVID-19 cases and demonstrated notable differences between the two investigated waves in the city of Tehran. As in the second wave, the mean age of patients was higher, and the frequency of smoking, opium abuse, and underlying diseases, particularly HTN, were more frequent than that of the first wave. The notable finding in this study is the significant increase in non-respiratory symptoms of patients in the second wave compared with the first wave. As expected, the report of probable contact with a COVID-19 patient has been increased in the second wave. Also, investigating variables such as cases with hypoxia, intubation, length of hospitalization, and death showed that the health status of patients who were transferred by ambulance during the first wave of the pandemic was worse than those transferred during the second wave.