1. Background
Metabolic syndrome (MetS), a collection of interconnected cardiovascular risk factors, is characterized by dysglycemia, hypertension, abdominal obesity, and dyslipidemia (1). Several diagnostic criteria have been used for MetS, including the International Diabetes Federation (IDF) criteria (2), the National Cholesterol Education Program Adult Treatment Panel III (NCEP-ATP III) criteria (3), and the revised NCEP-ATP III criteria, also known as the American Heart Association/National Heart, Lung, and Blood Institute (AHA/NHLBI) criteria (4). The prevalence of MetS can differ based on each set of criteria. The prevalence of MetS was about 34.7% based on NCEP-ATP III criteria, 37.4% based on IDF definition, and 41.6% based on AHA/NHLBI definition in Iran in 2007 (5). In this regard, IDF estimates the global prevalence of MetS as about 25% (6); however, this can vary widely based on age, gender, and ethnicity (7).
The presence of all MetS components increases the risk of cardiovascular morbidity and mortality compared to the presence of a single component (8, 9). Some MetS components are believed to be more important than others in that they can be independent predictors of new-onset of other MetS components. Abdominal obesity, defined by high waist circumference (WC) appears to be one of these components (10-12). Abdominal obesity has a negative effect on blood pressure (BP), triglyceride (TG) levels, fasting plasma glucose (FPG), and high-density lipoprotein (HDL) levels (1, 13). Therefore, in addition to the prevalence of MetS, investigating the prevalence of individual MetS components seems to be crucial.
Several factors are associated with MetS, including female gender, obesity, overweight, older age, urbanization, and low educational level. This contributes to the multifactorial nature of MetS (14, 15).
2. Objectives
As MetS has never been evaluated in this region of Iran and due to the unique characteristics of each population that can affect the frequency of MetS, in the current study, we aimed to determine the prevalence of MetS and its components in the PERSIAN Bandare-Kong Cohort study and evaluate the association of age, gender, place of residence, marital status, employment, education, and body mass index (BMI) on MetS and any individual MetS component.
3. Methods
3.1. Participants
We assessed the baseline data from participants of the Bandare-Kong Non-Communicable Diseases (BKNCD). In fact, BKNCD is within the Prospective Epidemiological Research Studies in IrAN (PERSIAN) Cohort in Bandare Kong, Iran, details of which have previously been described (16). The participants of this cohort study were 4,063 individuals aged 35 - 70 years that had been recruited between November 17, 2016, and November 22, 2018, from Bandare-Kong city, Hormozgan province, southern Iran. After excluding incomplete records and pregnant women, 3,927 individuals were included in the final analysis.
3.2. Study Design
A face-to-face interview was conducted by trained interviewers to collect sociodemographic data. Age, place of residence, education, marital status, and cigarette smoking were recorded. The socioeconomic status (SES) was separately estimated by multiple correspondence analysis (MCA), the counterpart of principal component analysis (PCA) for nominal categorical data. The analysis was constructed for the following variables: access to a freezer, access to a washing machine, a dishwasher, a computer, internet, a motorcycle, access to a vacuum cleaner, color TV type, owning a cell phone, a personal computer or a laptop, and international trips in a lifetime. According to their total asset score, subjects were ranked and then grouped into five quintiles, where the 1st quintile indicates the very low (the poorest) group and the 5th quintile indicates the very high (the richest). We combined the two upper and lower quantiles in this study to include enough participants in each category.
The daily ingested foods by the participants were recorded using the Food Frequency Questionnaire (FFQ), and based on their calorie content, the daily calorie intake was calculated. Moreover, by using the metabolic equivalent of tasks (METs), overall daily and weekly energy expenditure of each participant was determined.
Weight was measured using a standard digital scale (to the nearest 0.5 kg) while subjects were shoeless and had minimum clothing. Height was measured while subjects’ shoulders were set normally and as they were standing shoeless. After several natural breaths, a stretch-resistant tape was used to measure the midpoint circumference between the inferior margin of the last palpable rib and the top of the iliac crest. The results were recorded as waist circumference (WC). The average of two WC measurements was recorded for each participant.
The largest circumference of the buttocks was recorded as hip circumference (HC). The same stretch-resistance tape was used for all the measurements, and the results were recorded to the nearest 0.5 cm. During the measurement, participants were standing with their arms relaxed at the side, as well as evenly distributed body weight and evenly spread feet. By dividing WC by HC, waist-to-hip ratio (WHR) was calculated to the nearest 0.01.
Body mass index was calculated by dividing weight (kg) by the square of height (m). According to the World Health Organization (WHO) guidelines, BMI ≥ 30 was regarded as obese, 25 ≤ BMI < 30 as overweight, 18.5 ≤ BMI < 25 as normal, and BMI < 18.5 kg/m2 as underweight (17).
A standard mercury sphygmomanometer was used to measure blood pressure (BP) following five minutes of rest. The sphygmomanometer used had an appropriate cuff size. During BP measurement participants were in the seated position, arm supported at heart level, and feet on the floor (6). Two measurements were done at least five min apart, and their average was used for analysis.
Fasting plasma glucose was measured in venous blood samples collected after eight hours of fasting using the glucose oxidase method. Total cholesterol (TC), TG, low-density lipoprotien (LDL), and HDL were measured in a separate venous blood sample collected after 12 hours of fasting.
The cut-off value of WC ≥ 95 cm was used for both Iranian men and women based on the study by Azizi et al. (18). The Iranian National Committee of Obesity considered this cut-off as well as the following criteria for MetS, with any three out of five components qualifying a person for MetS (19):
(1) WC ≥ 95 cm
(2) FPG ≥ 100 mg/dL or drug treatment for elevated blood glucose
(3) HDL < 40 mg/dL in men, < 50 mg/dL in women, or drug treatment for low HDL
(4) TG ≥ 150 mg/dL or drug treatment for elevated TG
(5) BP ≥ 130/85 mmHg or drug treatment for hypertension
The study received ethics approval (ethics code: IR.HUMS.REC.1397.176) from the Ethics Committee of Hormozgan University of Medical Sciences, and it complies with the statements of the Declaration of Helsinki. Informed consent was obtained from all subjects and a parent and/or legal guardian for vulnerable population.
3.3. Data Analysis
We used the Statistical Package for the Social Sciences (SPSS) software (version 25.0, Armonk, NY: IBM Corp.) for data analysis. Qualitative variables were described using frequencies and percentages, while quantitative variables were described using means and standard deviations. The binary logistic regression model was used to determine the correlation of MetS with the associated factors. All the potential factors with P-values ≤ 0.2 in univariate correlations were simultaneously included in the multivariate logistic regression model using the “enter” method. The area under the receiver operating characteristic (ROC) curve was calculated to determine the prediction performance of the logistic regression model. P-values ≤ 0.05 were considered statistically significant. Of note, we used the direct standardization method to report the age-standardized prevalence of MetS.
4. Results
Of the 3,927 participants in this study, 2,230 (56.8%) were female. Age-standardized prevalence (ASP) of MetS was 26.3 (Iranian-specific criteria) to 35.4% (IDF criteria) in the study population based on different criteria for the diagnosis of MetS. Higher prevalence of MetS was observed in women (31.2 - 45.6%) compared to men (19.7 - 30.1%) (Table 1).
| Criteria | Total Population | Men | Women | |
|---|---|---|---|---|
| Crude | ASP | ASP | ASP | |
| NCEP | 36.9 (35.4 - 38.4) | 34.7 (33.3 - 36.1) | 24.1 (22.1 - 26.1) | 42.6 (40.6 - 44.5) |
| IDF | 37.3 (35.8 - 38.8) | 35.4 (33.9 - 36.8) | 21.8 (19.9 - 23.8) | 45.6 (43.6 - 47.5) |
| Iranian-specific criteria (NCEP) | 36.6 (35.1 - 38.1) | 34.5 (33.1 - 35.9) | 30.1 (27.9 - 32.3) | 37.6 (35.8 - 39.5) |
| Iranian-specific criteria (IDF) | 27.6 (26.2 - 29.0) | 26.3 (24.9 - 27.7) | 19.7 (17.8 - 21.6) | 31.2 (29.3 - 33.0) |
Crude and Age-Standardized Prevalence of MetS Based on Different Criteria
Mean age was significantly higher in women with MetS compared to men with MetS (P < 0.001). On the contrary, mean education years, weight, systolic blood pressure (SBP), diastolic blood pressure (DBP), TG, daily calorie intake, and physical activity were significantly higher in men with MetS compared to their female counterparts. Mean WC, HC, WHR, BMI, LDL, TC, and number of MetS components were significantly higher in women with MetS compared to men with MetS. Mean FPG was significantly higher in women with MetS compared to men with MetS. In general, mean age, weight, HC, WHR, BMI, and TC were significantly higher in those with MetS compared to those without MetS. Contrarily, mean education years, LDL, daily calorie intake, and physical activity were significantly higher in those without MetS. The highest prevalence of MetS was observed in those aged more than 55 - 59 years, living in urban areas, the married, with ≤ 6 years of education, the employed, non-smokers, the overweight, those with high SES, and those with low physical activity (Table 2).
| Variables | No MetS | P-Value* | MetS | P-Value* | Total | P-Value* | |||
|---|---|---|---|---|---|---|---|---|---|
| Men | women | Men | Women | No MetS | MetS | ||||
| Age (y) | 47.18 ± 9.19 | 45.79 ± 8.66 | < 0.001 | 50.87 ± 9.89 | 51.91 ± 8.92 | < 0.001 | 46.44 ± 8.93 | 51.52 ± 9.31 | < 0.001 |
| Weight (kg) | 71.38 ± 13.10 | 64.96 ± 13.19 | < 0.001 | 82.26 ± 13.93 | 73.24 ± 13.08 | < 0.001 | 67.96 ± 13.53 | 76.59 ± 14.09 | < 0.001 |
| WC (cm) | 87.30 ± 10.43 | 92.20 ± 11.29 | < 0.001 | 97.23 ± 9.30 | 102.41 ± 9.77 | < 0.001 | 89.92 ± 11.17 | 100.49 ± 9.92 | < 0.001 |
| HC (cm) | 95.99 ± 7.64 | 100.25 ± 9.87 | < 0.001 | 101.56 ± 8.09 | 104.53 ± 10.30 | < 0.001 | 98.26 ± 9.15 | 103.42 ± 9.65 | < 0.001 |
| WHR | 0.91 ± 0.06 | 0.92 ± 0.07 | < 0.001 | 0.96 ± 0.48 | 0.98 ± 0.06 | < 0.001 | 0.91 ± 0.06 | 0.97 ± 0.06 | < 0.001 |
| SBP (mmHg) | 116.97 ± 14.25 | 111.28 ± 14.55 | < 0.001 | 128.23 ± 18.31 | 126.24 ± 19.01 | < 0.001 | 113.93 ± 14.69 | 126.98 ± 18.77 | < 0.001 |
| DBP (mmHg) | 76.44 ± 9.31 | 73.00 ± 9.72 | < 0.001 | 82.62 ± 9.96 | 80.12 ± 10.31 | < 0.001 | 74.61 ± 9.68 | 81.05 ± 10.25 | < 0.001 |
| FPG (mg/dL) | 98.60 ± 29.84 | 95.40 ± 30.04 | < 0.001 | 121.21 ± 47.90 | 131.67 ± 57.00 | < 0.001 | 96.89 ± 29.98 | 127.78 ± 54.01 | < 0.001 |
| TC (mg/dL) | 198.71 ± 37.70 | 202.57 ± 40.88 | 0.014 | 199.66 ± 50.89 | 207.90 ± 44.30 | 0.004 | 200.77 ± 39.47 | 204.83 ± 47.01 | 0.006 |
| TG (mg/dL) | 124.13 ± 67.86 | 101.45 ± 42.94 | < 0.001 | 203.17 ± 149.11 | 165.14 ± 80.78 | < 0.001 | 112.04 ± 57.09 | 179.28 ± 112.67 | < 0.001 |
| HDL (mg/dL) | 46.38 ± 8.96 | 52.62 ± 10.81 | < 0.001 | 40.38 ± 9.64 | 47.09 ± 10.03 | < 0.001 | 49.71 ± 10.46 | 44.59 ± 10.40 | < 0.001 |
| LDL (mg/dL) | 127.84 ± 32.44 | 129.65 ± 34.06 | 0.177 | 120.18 ± 34.20 | 128.40 ± 37.55 | 0.425 | 128.81 ± 33.32 | 125.34 ± 36.54 | 0.003 |
| Physical activity (METS) | 293.05 ± 58.39 | 279.68 ± 31.17 | < 0.001 | 275.32 ± 42.09 | 272.30 ± 32.27 | < 0.001 | 285.92 ± 46.41 | 273.42 ± 36.25 | < 0.001 |
| Number of MetS components components | 1.12 ± 0.80 | 1.17 ± 0.76 | 0.151 | 3.43 ± 0.62 | 3.63 ± 0.71 | < 0.001 | 1.15 ± 0.78 | 3.56 ± 0.69 | < 0.001 |
| Age groups (y) | 0.006 | < 0.001 | < 0.001 | ||||||
| 35 - 39 | 302 (26.0) | 406 (30.6) | 93 (17.4) | 94 (10.4) | 708 (28.4) | 187 (13.0) | |||
| 40 - 44 | 232 (19.9) | 291 (21.9) | 82 (15.4) | 120 (13.3) | 523 (21.0) | 202 (14.1) | |||
| 45 - 49 | 214 (18.4) | 239 (18.0) | 70 (13.1) | 146 (16.2) | 453 (18.2) | 216 (15.0) | |||
| 50 - 54 | 140 (12.0) | 160 (12.0) | 73 (13.7) | 167 (18.5) | 300 (12.0) | 240 (16.7) | |||
| 55 - 59 | 125 (10.7) | 111 (8.4) | 82 (15.4) | 182 (20.2) | 236 (9.5) | 264 (18.4) | |||
| 60 - 64 | 94 (8.1) | 71 (5.3) | 87 (16.3) | 108 (12.0) | 165 (6.6) | 195 (13.6) | |||
| 65 - 70 | 56 (4.8) | 50 (3.8) | 47 (8.8) | 85 (9.4) | 106 (4.3) | 132 (9.2) | |||
| Place of residence | 0.526 | < 0.001 | 0.026 | ||||||
| Urban | 996 (46.4) | 1149 (53.6) | 469 (39.1) | 730 (60.9) | 2145 (64.1) | 1199 (35.9) | |||
| Rural | 167 (48.3) | 179 (51.7) | 65 (27.4) | 172 (72.6) | 346 (59.3) | 237 (40.7) | |||
| Marital status | < 0.001 | 0.022 | 0.001 | ||||||
| Single | 16 (23.9) | 51 (76.1) | 5 (19.2) | 21 (80.8) | 67 (72.0) | 226 (28.0) | |||
| Married | 1135 (50.5) | 1113 (49.5) | 524 (41.4) | 742 (58.6) | 2248 (64.0) | 1266 (36.0) | |||
| Widowed/Divorced | 12 (6.8) | 164 (93.2) | 5 (3.5) | 139 (96.5) | 176 (55.0) | 144 (45.0) | |||
| Education | < 0.001 | < 0.001 | < 0.001 | ||||||
| < 6 years | 498 (36.5) | 868 (63.5) | 254 (25.7) | 733 (74.3) | 1366 (58.1) | 987 (41.9) | |||
| 6 - 12 years | 529 (59.6) | 358 (40.4) | 213 (58.7) | 150 (41.3) | 887 (71.0) | 363 (29.0) | |||
| > 12 years | 136 (57.1) | 102 (42.9) | 67 (77.9) | 19 (22.1) | 238 (73.5) | 86 (26.5) | |||
| Occupation | < 0.001 | < 0.001 | < 0.001 | ||||||
| Employed | 1000 (80.0) | 250 (20.0) | 419 (82.0) | 92 (18.0) | 1250 (71.0) | 511 (29.0) | |||
| Unemployed | 163 (13.1) | 1078 (86.9) | 115 (12.4) | 810 (87.6) | 1241 (57.3) | 925 (42.7) | |||
| BMI categories | < 0.001 | < 0.001 | < 0.001 | ||||||
| Underweight | 57 (56.4) | 44 (43.6) | 3 (50.0) | 3 (50.0) | 101 (94.4) | 6 (5.6) | |||
| Normal | 599 (53.5) | 520 (46.5) | 100 (47.4) | 111 (52.6) | 1119 (84.1) | 211 (15.9) | |||
| Overweight | 408 (45.8) | 482 (54.2) | 258 (39.8) | 390 (60.2) | 890 (57.9) | 648 (42.1) | |||
| Obese | 99 (26.0) | 282 (74.0) | 173 (30.3) | 398 (69.7) | 381 (40.0) | 571 (60.0) | |||
| Smoking | 383 (99.2) | 3 (0.8) | < 0.001 | 186 (97.4) | 5 (2.6) | 0.282 | 386 (66.9) | 191 (33.1) | 0.061 |
| SES | < 0.001 | 0.788 | 0.981 | ||||||
| Very low | 173 (36.7) | 298 (63.3) | 59 (21.1) | 220 (78.9) | 471 (62.8) | 279 (37.2) | |||
| Low | 207 (43.3) | 271 (56.7) | 88 (31.7) | 190 (68.3) | 478 (63.2) | 278 (36.8) | |||
| Average | 251 (48.0) | 272 (52.0) | 118 (40.3) | 175 (59.7) | 523 (64.1) | 293 (35.9) | |||
| High | 266 (49.7) | 269 (50.3) | 135 (43.1) | 178 (56.9) | 535 (63.1) | 313 (36.9) | |||
| Very high | 266 (55.0) | 218 (45.0) | 134 (49.1) | 139 (50.9) | 484 (63.9) | 273 (36.1) | |||
| Low physical activity | 1159 (46.6) | 1328 (53.4) | 0.047 | 534 (37.2) | 902 (62.8) | - | 2487 (63.4) | 1436 (36.6) | 0.304 |
Sociodemographic and Anthropometric Features of Participants Based on the Presence of MetS According to Iranian-specific Definition (N = 3,927) a
The most common component of MetS in this population was central obesity (45.1%), followed by decreased HDL (42.6%), elevated FPG (39.9%), elevated TG (37.7%), and elevated BP (37.5%). The prevalence of MetS components based on different sociodemographic characteristics is summarized in Table 3.
| Variables | Central Obesity | Elevated FPG | Elevated TG | Decreased HDL | Elevated BP |
|---|---|---|---|---|---|
| Age (y) | |||||
| 35 - 39 | 350 (39.1) | 202 (22.6) | 248 (27.7) | 383 (42.8) | 147 (16.4) |
| 40 - 44 | 319 (44.0) | 222 (30.6) | 234 (32.3) | 340 (46.9) | 149 (20.6) |
| 45 - 49 | 301 (45.0) | 249 (37.2) | 226 (33.8) | 305 (45.6) | 224 (33.5) |
| 50 - 54 | 267 (49.4) | 270 (50.0) | 237 (43.9) | 218 (40.4) | 251 (46.5) |
| 55 - 59 | 256 (51.2) | 286 (57.2) | 247 (49.4) | 191 (38.2) | 290 (58.0) |
| 60 - 64 | 166 (46.1) | 200 (55.6) | 171 (47.5) | 141 (39.2) | 230 (63.9) |
| 65 - 70 | 111 (46.6) | 136 (57.1) | 119 (50.0) | 96 (40.3) | 181 (76.1) |
| P-value | < 0.001 | < 0.001 | < 0.001 | 0.019 | < 0.001 |
| Gender | |||||
| Male | 574 (33.8) | 666 (39.2) | 718 (42.3) | 539 (31.8) | 639 (37.7) |
| Female | 1196 (53.6) | 899 (40.3) | 764 (34.3) | 1135 (50.9) | 833 (37.4) |
| P-value | < 0.001 | 0.498 | < 0.001 | < 0.001 | 0.847 |
| Place of residence | |||||
| Urban | 1486 (44.4) | 1254 (37.5) | 1258 (37.6) | 1508 (45.1) | 1189 (35.6) |
| Rural | 284 (48.7) | 311 (53.3) | 224 (38.4) | 166 (28.5) | 283 (48.5) |
| P-value | 0.056 | < 0.001 | 0.712 | < 0.001 | < 0.001 |
| Marital status | |||||
| Single | 33 (35.5) | 32 (34.4) | 24 (25.8) | 37 (39.8) | 24 (25.8) |
| Married | 1571 (44.7) | 1385 (39.4) | 1335 (38.0) | 1482 (42.2) | 1292 (36.8) |
| Widowed/divorced | 166(51.9) | 148 (46.3) | 123(38.4) | 155 (48.4) | 156(48.8) |
| P-value | 0.008 | 0.032 | 0.055 | 0.081 | < 0.001 |
| Education | |||||
| Illiterate | 854 (49.2) | 838 (48.2) | 698 (40.2) | 763 (43.9) | 886 (51.0) |
| Literate | 916 (41.8) | 727 (33.2) | 784 (35.8) | 911 (41.6) | 586 (26.8) |
| P-value | < 0.001 | < 0.001 | 0.005 | 0.143 | < 0.001 |
| Occupation | |||||
| Employed | 618 (35.1) | 638 (36.2) | 675 (38.3) | 618 (35.1) | 540 (30.7) |
| Unemployed | 1152 (53.2) | 927 (42.8) | 807 (37.3) | 1056 (18.8) | 932 (43.0) |
| P-value | < 0.001 | < 0.001 | 0.490 | < 0.001 | < 0.001 |
| BMI | |||||
| Underweight | 0 (0.0) | 12 (11.2) | 15 (14.0) | 27 (25.2) | 13 (12.1) |
| Normal | 67 (5.0) | 442 (33.2) | 367 (27.6) | 459 (34.5) | 396 (29.8) |
| Overweight | 796 (51.8) | 658 (42.8) | 661 (43.0) | 722 (46.9) | 627 (40.8) |
| Obese | 907 (95.3) | 453 (47.6) | 439 (46.1) | 466 (48.9) | 436 (45.8) |
| P-value | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
| Smoking | |||||
| Yes | 184 (31.9) | 216 (37.4) | 250 (43.3) | 207 (35.9) | 226 (39.2) |
| No | 1586 (47.3) | 1349 (40.3) | 1232 (36.8) | 1467 (43.8) | 1246 (37.2) |
| P-value | < 0.001 | 0.199 | 0.003 | < 0.001 | 0.366 |
| SES | |||||
| Very low | 332 (44.3) | 295 (39.3) | 260 (34.7) | 358 (47.7) | 292 (38.9) |
| Low | 335 (44.3) | 294 (38.9) | 288 (38.1) | 336 (44.4) | 283 (37.4) |
| Average | 358 (43.9) | 316 (38.7) | 298 (36.5) | 363 (44.5) | 298 (36.5) |
| High | 401 (47.3) | 352 (41.5) | 327 (38.6) | 337 (39.7) | 323 (38.1) |
| Very high | 344 (45.4) | 308 (40.7) | 309 (40.8) | 280 (37.0) | 276 (36.5) |
| P-value | 0.632 | 0.736 | 0.143 | < 0.001 | 0.832 |
| MetS | |||||
| Yes | 1084 (61.2) | 1053 (67.3) | 1061 (41.6) | 917 (54.8) | 993 (67.5) |
| No | 686 (38.8) | 512 (32.7) | 421 (28.4) | 757 (45.2) | 479 (32.5) |
| P-value | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
| Total | 1770 (45.1) | 1565 (39.9) | 1482 (37.7) | 1674 (42.6) | 1472 (37.5) |
Prevalence of MetS Components by Sociodemographic Characteristics According to Iranian-specific Definition Among Population (N = 3,927) a
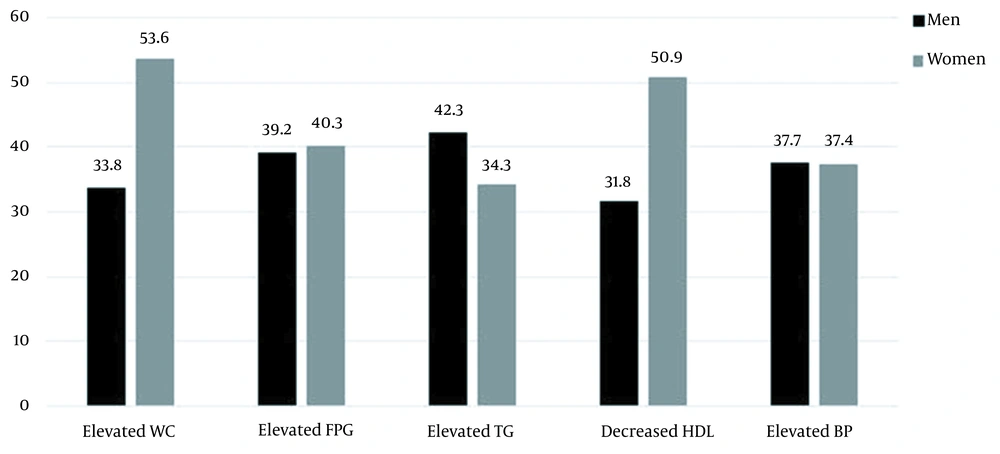
The most prevalent component in women with MetS was central obesity (53.6%), followed by decreased HDL (50.9%) and dysglycemia (40.3%), while hypertriglyceridemia (42.3%) and raised BP (37.7%) were the most common components in their male counterparts (Figure 1). According to the age- and gender-categorized analysis, reduced HDL, abdominal obesity, and elevated TG were the more common elements in younger females, while hypertriglyceridemia, raised WC, and reduced HDL were common in younger men. Meanwhile, high BP and dysglycemia were more common in the elderly population compared to younger adults in both genders. In addition, a significant increase was observed in high BP and dysglycemia from 35 to 70 years of age in both genders (Appendices 1 and 2 in Supplementary File).
Table 4 shows the number of MetS components based on different characteristics of the study population. Among the participants, 15.3% had no components of MetS, 23.7% had only one component, 24.5% had two components, 20.3% had three, 12.2% had four, and 4.1% had all of the MetS components. Besides, 84.7% of the subjects displayed at least one component of MetS. Females, the widowed/divorced, the illiterate, rural residents, the unemployed, and non-smokers showed a higher number of MetS components compared to their counterparts (Appendix 3 in Supplementary File).
| Variables | Men | Women | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| B | S.E. | P-Value | OR | 95% CI | B | S.E. | P-Value | OR | 95% CI | |
| Age (y) | ||||||||||
| 35 - 39 a | ||||||||||
| 40 - 44 | 0.234 | 0.194 | 0.229 | 1.26 | 0.86 - 1.85 | 0.556 | 0.171 | 0.001 | 1.74 | 1.25 - 2.44 |
| 45 - 49 | 0.307 | 0.206 | 0.135 | 1.36 | 0.91 - 2.04 | 0.881 | 0.174 | < 0.001 | 2.41 | 1.72 - 3.39 |
| 50 - 54 | 0.839 | 0.219 | < 0.001 | 2.31 | 1.51 - 3.56 | 1.568 | 0.182 | < 0.001 | 4.80 | 3.36 - 6.85 |
| 55 - 59 | 1.233 | 0.230 | < 0.001 | 3.43 | 2.19 - 5.38 | 2.110 | 0.194 | < 0.001 | 8.25 | 5.64 - 12.07 |
| 60 - 64 | 1.614 | 0.244 | < 0.001 | 5.02 | 3.12 - 810 | 2.234 | 0.226 | < 0.001 | 9.34 | 6.00 - 14.54 |
| 65 - 70 | 1.56 | 0.296 | < 0.001 | 4.75 | 2.66 - 8.49 | 2.516 | 0.253 | < 0.001 | 12.37 | 7.54 - 20.30 |
| Education years | -0.004 | 0.014 | 0.783 | 0.996 | 0.969 - 1.024 | -0.037 | 0.015 | 0.014 | 0.964 | 0.936 - 0.993 |
| Place of residence | ||||||||||
| Urban a | ||||||||||
| Rural | 0.042 | 0.179 | 0.814 | 1.04 | 0.73 - 1.48 | 0.435 | 0.138 | 0.002 | 1.55 | 1.18 - 2.03 |
| Marital status | ||||||||||
| Single a | ||||||||||
| Married | -0.163 | 0.596 | 0.784 | 0.85 | 0.26 - 2.73 | -0.451 | 0.320 | 0.159 | 0.64 | 0.34 - 1.19 |
| Widowed/divorced | -0.566 | 0.847 | 0.504 | 0.57 | 0.11 - 2.99 | -0.0653 | 0.349 | 0.061 | 0.52 | 0.26 - 1.03 |
| Occupation | ||||||||||
| Unemployed a | ||||||||||
| Employed | -0.023 | 0.174 | 0.897 | 0.98 | 070 - 1.37 | -0.323 | 0.156 | 0.038 | 0.72 | 0.53 - 0.98 |
| BMI | ||||||||||
| Underweight and normal a | ||||||||||
| Overweight | 1.57 | 0.142 | < 0.001 | 4.80 | 3.63 - 6.34 | 1.659 | 0.138 | < 0.001 | 5.26 | 4.01 - 6.89 |
| Obese | 2.813 | 0.182 | < 0.001 | 16.66 | 11.65 - 23.81 | 2.344 | 0.147 | < 0.001 | 10.43 | 7.82 - 13.90 |
| Smoking status | ||||||||||
| No a | ||||||||||
| Yes | -0.076 | 0.130 | 0.558 | 0.93 | 0.72 - 1.20 | -1.173 | 0.769 | 0.127 | 0.31 | 0.07 - 1.40 |
| SES | ||||||||||
| Very low a | ||||||||||
| Low | 0.157 | 0.224 | 0.485 | 1.17 | 0.75 - 1.82 | -0.065 | 0.151 | 0.665 | 0.94 | 0.70 - 1.26 |
| Average | 0.228 | 0.215 | 0.289 | 1.26 | 0.82 - 1.91 | -0.114 | 0.153 | 0.456 | 0.89 | 0.66 - 1.21 |
| High | 0.100 | 0.214 | 0.640 | 1.11 | 0.73 - 1.68 | -0.138 | 0.155 | 0.373 | 0.87 | 0.64 - 1.18 |
| Very high | 0.039 | 0.219 | 0.859 | 1.04 | 0.68 - 1.60 | -0.133 | 0.169 | 0.430 | 0.88 | 0.63 - 1.22 |
Logistic Regression Analysis of MetS by Sociodemographic Characteristics (N = 3,927)
Binary logistic regression revealed that the odds of MetS significantly increased with age in women; those aged 65 - 70 years were almost 12.5 folds at higher risk of having MetS compared to those aged 35-39 years (OR = 12.37, 95% CI 7.54 - 20.30, P < 0.001). Yet, this was not the case for men; although the odds of MetS increased from 50 to 64 years of age, there was a slight decrease in the 65 - 70 years age group. In addition, living in rural areas was a risk for MetS in women (OR = 1.55, 95% CI 1.18 - 2.03, P = 0.002), while the place of residence had no role in the presence of MetS in men. Being employed and educated were protective against MetS in women (OR = 0.72, 95% CI 0.53 - 0.98) and (OR = 0.96, CI: 0.936 - 0.993), then again it did not influence the presence of MetS in men. Being overweight and obese significantly increased the risk of MetS in both men and women. Obesity was more problematic for men (OR = 16.66, 95% CI 11.65 - 23.81, P < 0.001) compared to women (OR = 10.43, 95% CI 7.82 - 13.90, P < 0.001). Marital status, education, smoking status, and SES did not significantly predict the presence of MetS (Table 4).
The area under the ROC curve of the logistic regression model for prediction of MetS was 0.785 (95% CI: 0.771 - 0.800), which showed the acceptable performance of this model.
5. Discussion
This study demonstrates the first prevalence estimation of MetS amid general population in a southern coastal area of Iran. Our study showed an age-standardized prevalence of 26.3 - 35.4% for MetS based on different criteria in a population aged 35 - 70 years. The IDF criterion for abdominal obesity emphasizes population-specific WC; however, according to the report of the Iranian National Committee of Obesity (19), the harmonized definition of MetS using identical cut-offs of WC for both genders and requiring any three components with no obligatory component for the diagnosis of MetS appears to be more appropriate for the Iranian population and was used in the current study for further analysis.
One of the largest studies in the United States, analyzing data from the National Health and Nutrition Examination Survey (NHANES), showed that using the harmonized criteria for MetS, more than one-third of US adults met the criteria by 2012 (20). In a recent meta-analysis of 69 studies carried out in Iran, the overall prevalence of MetS in adults over 20 years of age was 30.4% (10). The prevalence of MetS in the same age group and criteria in the Iranian population was 33.1% (21), which is close to our results (34.5%). The prevalence of MetS has also been reported in other parts of the world, including 28.8% in Turkey (22), 25.9% in Denmark (23), 5.3% in Japan (24), 31.2% in Lebanon (25), 32% in Brazil (26), and 24.5% in China (27). The variability of these reports can be due to the different diagnostic criteria used for MetS, different age of the studied populations, nutrition, physical activity, and general lifestyle.
The high prevalence of MetS, given its potential consequences, calls for the identification of any etiological factors that may contribute to the development of MetS. Thus, we assessed the frequency of MetS components and the effect of sociodemographic factors such as age, gender, marital status, education, occupation, place of residence, SES, anthropometric indices, concentration of some serum elements, blood pressure, calorie intake, and physical activity on the presence of MetS in the study population. Furthermore, population-specific studies are very valuable, since there may be some unmeasurable factors involved that differ across populations.
Regarding the prevalence of individual components, central obesity, followed by decreased HDL, elevated FPG, hypertriglyceridemia, and raised BP were the most prevalent in our study population. The most important component of MetS is abdominal obesity, a metabolically active adipose tissue (28) associated with insulin resistance. Besides, it plays a critical role in MetS consequence (29). The results of a meta-analysis on the Iranian population (10), a study in south of Iran (21), as well as a Turkic ethnic study in Iran (30), demonstrated consistent findings in which abdominal obesity was the most common component. However, a study in southeastern Iran found hypertriglyceridemia as the dominant component (31). Ostovar et al. reported low HDL and high TG as more prevalent components among adult population (32). This might be explained by the difference in the age of the population, physical activity, and nutritional issues.
We found that participants with MetS were significantly older than those without MetS. Our study revealed that the odds of MetS increased quite steadily with age in women, having the same trend in men but only from 50 to 64 years of age, with a slight decrease after the age of 64. More than 80% of MetS subjects were over 45 years of age in our study. Also, a Northern Indian study results revealed that more than 80% of MetS population were over 40 years (33). Moreover, women with MetS were significantly older than men with MetS. Besides, the older subjects had a higher number of components, and dysglycemia and high BP were more prevalent among older patients. Similarly, in the study by Moore et al., advanced age significantly increased the odds of MetS (20). Also, in line with the findings of our study, in a 12-year cohort of Iranian adults, the prevalence of MetS increased up to the age of 75 (10). The increased prevalence of MetS among older adults could be justified by age-dependent hormonal alterations in insulin and counter-regulatory hormones as well as increased sedentary lifestyle in older adults, probably due to functional disabilities (34, 35).
Our study showed that the prevalence of MetS was higher in women (37.6%) compared to men (30.1%). This result is in agreement with the findings of a recent meta-analysis of Iranian studies (10), the study by Nikbakht et al. (21), and Jahangiry et al.’s research (30). Our findings demonstrated that the prevalence of MetS components in MetS subjects, as well as their number, were higher in women than men in all age categories. Women more frequently presented central obesity and dysglycemia, while in men, hypertriglyceridemia and dysglycemia were more frequent. This was different in younger subjects. Although abdominal obesity and reduced HDL were the most common elements in younger females, hypertriglyceridemia and elevated WC were the most common in younger men. High BP and dysglycemia were the most common components in the elderly population compared to younger adults in both genders. In addition, these components had significant growth between 35 and 70 years of age. An increasing trend in abdominal obesity was observed up to 60 years of age in women; however, the increasing trend of abdominal obesity was relatively steady in men across all age groups. Kalan Farmanfarma et al. and Nikbakht et al. reported similar findings (10, 21) with regard to the frequency of MetS components.
Gender has a fundamental effect on MetS risk factors and components such as dyslipidemia, dysglycemia, and central obesity (36). The gender effect is a consequence of the difference in the distribution of adipose tissue between men and women. It is demonstrated that IFG is more common in men, especially in older age groups; conversely, impaired glucose tolerance (IGT) is more frequent in women. On the other hand, the global prevalence of obesity is higher in women compared to men (37). Although the precise mechanisms of gender differences remain to be fully elucidated, the amount and distribution of adipose tissue, different patterns of insulin resistance, sex hormone alterations during menopause, and nutritional factors have been proposed as possible reasons (38). In addition, different genetic background, socio-demographic characteristics, physical activity status, and nutritional factors can influence the gender differences regarding the prevalence of MetS and its components.
In our study, logistic regression showed a significant association between education and MetS in women. This is in agreement with the findings of previous studies. Dutra et al. demonstrated that education can be a protective factor against MetS in women (26).
Also, we found that living in rural areas was associated with MetS in women, which is in agreement with the Asian population studies. They found that rural residents consume less vegetables and having a high-calorie diet (39, 40). Other studies reported that a vegetable-rich diet was associated with a lower risk of MetS and its components, including high BP and hyperglycemia (41, 42). However, we did not have the details of dietary components in our study. Contrarily, other studies demonstrated a higher frequency of MetS in urban-dwelling subjects compared to our study (43, 44).
One interesting issue is that in the current study, 71.8% of women had < 6 years of education (in comparison with 44.3% in men). It might be due to cultural issues affecting the degree of education by gender. Moreover, every one more year of education decreased the risk of MetS by nearly 5% in illiterate women. Nikbakht et al. also reported the same results (21).
Although the prevalence of MetS was higher in those with average and high SES, there was no significant association between SES and MetS in our study. This was contrary to the findings of Zuo et al., who reported that high income was positively associated with MetS risk (45). Moore et al. also found contradictory results; low SES was strongly associated with MetS in their study (20). Our study was conducted as the first large population-based study in Hormozgan province, a south coastal area of Iran, by valid and precise measurements and protocols. A large sample size is another privilege of the study. One limitation of our study was its cross-sectional design. Furthermore, over 90% of the participants had low physical activity at baseline, which could not be an effective indicator of physical activity for comparison between subjects with and without MetS. Besides, we did not have the complementary nutritional information at the time of analysis.
Given the high prevalence of MetS and its components (central obesity, high TG, and low HDL), especially in younger individuals, all considered as cardiovascular risk factors, it will be critical for research studies to focus on identifying etiological factors and for governments to work on prevention strategies for MetS. Population-specific studies such as ours are essential for identifying groups of people at higher risk of MetS for which tailored disease management strategies may be needed. A good strategy to prevent MetS would be to identify individuals with a lower number of abnormal MetS components than what is required for the diagnosis of MetS, for instance, those with only one component of MetS, and then take measures to avoid an increase in abnormal components or the development of MetS.