1. Background
Based on 2010 statistics, prostate cancer is the most frequent cancer among men, with the highest incidence (28%) and the second most important cause of mortality (11%) after lung cancer (29%) (1). Prostate cancer is responsible for 10% of cancer mortality in the United States. The incidence of prostate cancer varies greatly between countries and populations of different races, ranging from 4 to 7 per 100,000 populations in Asian countries to 70 - 100 per 100,000 populations in European and North American countries, and some studies have shown differences of up to 90 times from one geographical area to another (2). Due to its increasing trend, the share of this cancer in relation to all cancers in the country is increasing (3). The PSA is currently most widely used in combination with the Gleason score (GS) in the diagnosis of prostate cancer, with limitations including false-positive results. Patients with grades 2 - 4 never die from prostate cancer, but most patients with grades 8 - 10 die from the tumor (4, 5). The current approach to diagnosing prostate adenocarcinoma is that if the total serum PSA is more than 4 ng/mL, the patient should have a rectal examination and ultrasound followed by a biopsy if necessary (6). The combination of the rectal examination, anal ultrasound, and serum PSA is a valuable diagnostic triangle for early detection of prostate cancer, but pathological confirmation is always necessary (7).
IHC is used as a prognostic or predictive marker, as an implement in exploring new therapeutic targets, and even in combination as a replacement for molecular classifications and multigene prognostic panels (8). The severity of the PSA marker position in the cytoplasm of cells. Most studies have shown that higher serum PSA levels are associated with higher Gleason grade and are inversely related to the severity of PSA marker staining by immunohistochemistry. Due to the prevalence of prostate adenocarcinoma and the importance of measuring serum PSA in screening for the disease and its comparison with Gleason grade (4), the present study was performed to evaluate the comparison between the degree of staining of PSA marker and some factors in the prognosis of prostate cancer.
2. Methods
2.1. Case Study and Sample Collection
This analytical cross-sectional study was on prostate adenocarcinoma, which was selected from the pathology department, at Imam Khomeini Hospital, Ahvaz, Iran, between 2019 and 2020. Hematoxylin-eosin stained slides were studied. Patients with prostate adenocarcinoma, sufficient tumor mass, presence of pathological slides of lymph nodes, and perfect medical records were the inclusion criteria. Exclusion criteria include not measuring preoperative PSA and cases of elevated serum PSA levels other than prostate adenocarcinoma, including prostate inflammation, prostate infarction, and preoperative urinary catheterization. The total number of paraffin samples of prostate adenocarcinoma from 2019 to 2020 was used as a sample size. According to the inclusion criteria, 97 formalin-embedded paraffin samples were included in the study. Clinical information, including age, tumor size, grade of tumor, lymph node involvement, and vascular and perineural invasion, was extracted from patients' pathology reports and recorded on a checklist.
2.2. Immunohistochemical Test
Immunohistochemical tests were performed according to the protocols used in previous studies (5, 9). This study used 1 μg/mL diluted PSA primary anti-mouse monoclonal antibody (clone RTU-PSA-28A4 Leica/Novocastra). Simultaneously with the staining of the main slide, a positive control (normal prostate tissue) was used. Internal control of non-glandular tissue was used for negative control. Positive staining for PSA was interpreted as the presence of brown spots on the cytoplasm of cells. The percentage of cells stained brown compared to cells stained blue was evaluated in each tissue. Apical PSA staining was scored as positive or negative. Also, the intensity of staining of the PSA marker based on the degree of cytoplasmic of stained cells was scored to 4 grade zero to +4 as follows:
• Grade 0: The cell is not stained.
• Grade 1: 1 - 5% staining of cells.
• Grade 2: 6 - 25% staining of cells.
• Grade 3: 26 - 75% staining of cells.
• Grade 4: Staining of more than 75% of cells
2.3. Statistical Analysis
For qualitative variables, frequency, percentage, and descriptive statistics, including mean index and standard deviation, were used for quantitative variables. In the analytical part, the data were analyzed using chi-square, t-test, Spearman, and other related tests. Significance level P < 0.05, and all analyzes were done using SPSS software version 25.
2.4. Ethics
The study was approved by the Ethics Committee of Ahvaz Jundishapur University of Medical Sciences (IR.AJUMS.HGOLESTAN.REC.1399.159).
3. Results
The mean age of patients with prostate cancer was 72.76 ± 8.19 years. The mean serum PSA level was 39.2 ± 8.90. Demographic and clinical information of patients is presented in Table 1.
| Variables | No. (%) |
|---|---|
| Gleason score | |
| 7.00 | 38 (39.2) |
| 8.00 | 20 (20.6) |
| 9.00 | 39 (40.2) |
| PSA marker staining intensity | |
| Grade 1 | 39 (40.2) |
| Grade 2 | 9 (9.3) |
| Grade 3 | 11 (11.3) |
| Grade 4 | 38 (39.2) |
| PSA marker apical staining | |
| Positive | 58 (58.9) |
| Negative | 39 (40.2) |
| Lymphovascular invasion | |
| No | 84 (86.6) |
| Yes | 13 (13.4) |
| Perineural invasion | |
| No | 29 (29.9) |
| Yes | 68 (70.1) |
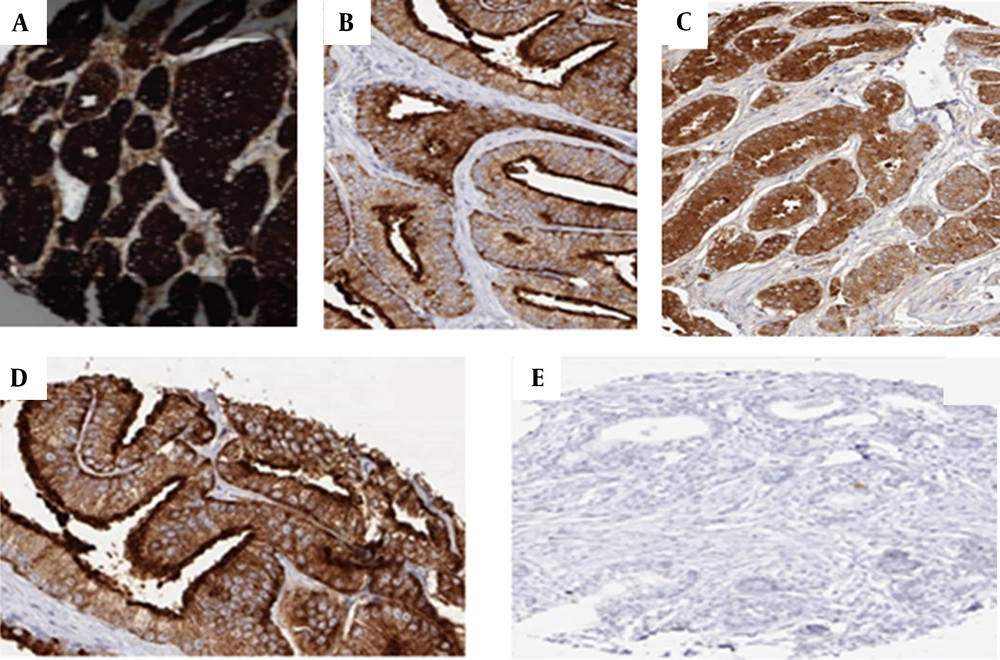
In the present study, the highest PSA staining intensity in the samples was related to grade 1 with a frequency of 39 (40.2%), and then it was related to grade 4 with a frequency of 38 (39.2%), while the lowest frequency of staining intensity was related to grade 2 was with a frequency of 9 (9.3%). Regarding PSA apical staining, 58 samples (59.8%) were positive, and 39 samples (40.2%) were negative (Figure 1).
Representative of PSA immunostaining in prostate cancer. IHC staining for PSA of prostate cancer was evaluated in apical staining and staining intensity. From the above left: (A) Normal prostate tissue with intensity staining grade 4; (B) Normal prostate tissue with positive apical staining and staining intensity grade 3; (C) Prostate tumor tissue with negative apical staining and staining intensity grade 3; (D) Prostate tumor tissue with negative apical staining and staining intensity grade 2; (E) Prostate tumor tissue with negative apical staining and intensity staining grade 1.
In the present study, all samples with Gleason 7 score were positive for PSA apical staining; on the other hand, all samples with Gleason 9 score were negative for PSA apical staining. Also, in samples with a Gleason score of 8, 19 samples (95%) were negative for PSA apical staining, and only one positive sample (5%) was positive. In general, a statistically significant comparison was shown between Gleason scoring and PSA apical staining (P < 0.001, Table 2).
| Variables | PSA Marker Apical Staining (%) | P-Value | PSA Marker Staining Intensity (%) | P-Value | ||||
|---|---|---|---|---|---|---|---|---|
| Negative | Positive | Grade 1 | Grade 2 | Grade 3 | Grade 4 | |||
| Gleason score | < 0.001 | < 0.001 | ||||||
| 7 | 0 (0.0) | 38 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 38 (100) | ||
| 8 | 19 (95.0) | 1 (5.0) | 0 (0.0) | 9 (45) | 11 (55) | 0 (0.0) | ||
| 9 | 39 (100.0) | 0 (0.0) | 39 (100) | 0(0.0) | 0(0.0) | 0 (0.0) | ||
| Lymphovascular invasion | 0.92 | 0.289 | ||||||
| Negative | 53 (63.1) | 31 (36.9) | 35 (41.7) | 9 (10.7) | 10 (11.9) | 30 (35.7) | ||
| Positive | 5 (38.5) | 8 (61.5) | 4 (30.8) | 0 (0.0) | 1 (7.7) | 8 (61.5) | ||
| Perineural invasion | 0.29 | 0.199 | ||||||
| Negative | 15 (51.7) | 14 (48.3) | 7 (24.1) | 4 (13.8) | 4 (13.8) | 14 (48.3) | ||
| Positive | 43 (63.2) | 25 (36.8) | 32 (47.1) | 5 (7.4) | 7 (10.3) | 24 (35.3) | ||
Regarding the intensity of PSA staining, all 38 specimens with a Gleason score of 7 had Grade 4 staining, while for specimens with a Gleason score of 8, 11 specimens (55%) had Grade 3, and 9 specimens (45%) had Grade 2. Even with 39 specimens with a Gleason score of 9, all specimens were graded 1 for staining intensity.
Based on the Kendall correlation coefficient test, the value of the correlation coefficient for the comparison between two Gleason scoring variables with PSA marker staining intensity was 0.984, which indicates that with increasing Gleason scoring, the staining intensity decreases and this correlation is significant (P = 0.001, Table 3).
| PSA Marker Apical Staining | Number | Mean ± SD | Standard Error | 95% (Confidence Interval) | P-Value | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Age | 0.195 | 6.81 | 0.38 | |||
| Negative | 58 | 74.1724 ± 8.89 | 1.168 | |||
| Positive | 39 | 70.6667 ± 6.58 | 1.05473 | |||
| Serum PSA | - 16.46 | - 11.90 | 0.000 | |||
| Negative | 58 | 33.5862 ± 5.29 | 0.69569 | |||
| Positive | 39 | 47.7692 ± 5.91 | 0.94695 | |||
t-Test results show that the mean age in samples with positive staining of apical marker PSA is lower than in samples with negative staining of apical marker PSA but is not statistically significant (P = 0.38). However, the serum level of the PSA marker is statistically significantly higher in samples with positive staining of apical marker PSA than in samples with negative staining of PSA (P < 0.001, Table 3).
According to the Spearman correlation coefficient test, the intensity of staining of the PSA marker decreases with age, and this correlation is significant (P = 0.032). However, according to the Spearman correlation coefficient test, staining intensity increases with increasing serum PSA level, and this correlation is statistically significant (P < 0.001).
Based on the results in specimens with lymphovascular invasion, the highest frequency in terms of PSA staining intensity was related to Grade 4 with a frequency of 61.5%, and the lowest frequency was related to Grade 2 with a frequency of zero.
While in samples with negative lymphovascular invasion, the highest frequency in terms of intensity of staining of PSA marker was related to grade 1 with a frequency of 35 (41.7%), and the lowest frequency was related to grade 2 with a frequency of 9 (10.7%). The chi-square test presented that there was no statistically significant comparison between lymphovascular invasion and intensity of staining of the PSA marker (P = 0.289).
In 68 samples with positive perineural invasion, the highest frequency in terms of intensity of staining of the PSA marker was related to grade 1 with a frequency of 32 (47.1%), and the lowest frequency was related to grade 2 with a frequency of 5 (7.4%). While in 29 samples with negative perineural invasion, the highest frequency in terms of intensity of staining of PSA marker was related to grade 4 with a frequency of 14 (48.3%), and the lowest frequency was related to grade 2 and 3 with a frequency of 4 (13.8%). The chi-square test showed no statistically significant comparison between perineural invasion and intensity of staining of the PSA marker (P = 0.199, Table 2).
The results show that in 13 samples with positive lymphovascular invasion, 8 samples (61.5%) were positive for PSA apical staining. While in 84 samples with negative lymphovascular invasion, 31 samples (36.9%) were positive for PSA apical staining. The chi-square test showed no statistically significant comparison between lymphovascular invasion and PSA apical staining (P = 0.92, Table 2).
The results show that in 68 samples with perineural invasion, 25 samples (36.8%) were positive for PSA apical staining. While in 29 samples with negative perineural invasion, 14 samples (48.3%) were positive for PSA apical staining. The chi-square test displayed no statistically significant comparison between perineural invasion and PSA apical staining (P = 0.290).
4. Discussion
The present study compared Gleason grade, serum PSA staining intensity, and apical staining of the PSA marker in prostate cancer. In the present study, most patients had a Gleason score of 9 with a frequency of 39 (40.2%) and then a score of 7 with a frequency of 38 (39.2%), and the lowest frequency referred to Gleason 8 with a frequency of 20 (20.6%). The highest staining intensity of the PSA marker in the samples was related to Grade 1 with a frequency of 39 (40.2%) and then to Grade 4 with a frequency of 38 (39.2%), while the lowest frequency of staining intensity was assigned to grade 2 with a frequency of 9 (9.3%). For apical PSA staining, 58 specimens (59.8%) were positive, and 39 specimens (40.2%) were negative.
In general, the results of the present study showed that as the Gleason score increases, the rate of positive apical markers and the staining intensity of the PSA marker decrease, which is statistically significant. On the other hand, the results of this study showed that the serum level of the PSA marker was statistically significantly higher in samples with positive apical PSA staining than in samples with apical PSA staining. The PSA marker staining intensity also increases significantly with increasing serum PSA levels. This question has been examined in previous studies, and the results of some studies are consistent with the results of the present study, and some results are inconsistent with the present study.
Farajian Abbasi et al. evaluated the PSA marker staining intensity using an immunohistochemical technique. Results showed that tumor grading ranged from 2 to 10, and the highest frequency of grading was associated with grade 7, and as the grade of PSA staining decreased, serum PSA levels gradually increased (P = 0.017) (10).
A study by Qin Xiao et al. showed that as Gleason scoring increases, serum PSA levels increase, and positive immunohistochemical staining of PSA tumors becomes weaker. In general, their results showed that the Gleason score was directly related to the serum PSA level and inversely related to the staining intensity of the PSA marker (11).
The study by Lakhtakia et al. showed that tissue PSA staining was strong (+4) in benign lesions (except atrophic glands). Serum PSA levels increased with Gleason scoring, while staining decreased (12).
Based on previous studies, PSA measurements provide useful information about prognosis in patients with prostate cancer, in addition to the high sensitivity and specificity known for prostate epithelial tissue (6). Few studies have compared PSA marker staining intensity and clinicopathological factors in prostate cancer. Staining intensity and signal-to-noise ratio depend significantly on the type of reagents and experimental protocol used. Therefore, data from previous immunohistochemical expression studies for most proteins analyzed by different research groups are very variable (13, 14). The selected experimental method expresses a range below which all staining will be "negative" and above which all staining results will be "strongly positive." If staining causes "dark brown" tissue elements, a concentration ten times higher than the protein of interest will no longer lead to stronger staining (15). In general, recent studies show that the level of PSA expression in prostate cancer cells is one of the strongest prognostic factors in this tumor. On the other hand, the reason why tumors are more aggressive in cancers with reduced PSA expression is not clear. Researchers have suggested a protective role for PSA in tumors (16). However, PSA production may be one of the most important functions of normal prostate cells. Therefore, it can be speculated that the lack of measurement of this function could be a sign of facile cell differentiation. Normal prostate glands have strong PSA staining at the apical cell border. Loss of this physiological function of apical PSA staining is directly linked to poor prognosis (17). Successful analysis of more than 12,000 prostate cancers showed that even when undifferentiated (Gleason score greater than 8), more than 99% of prostate cancers expressed PSA at levels detectable at higher antibody concentrations (15). Previous studies have shown that one of the reasons why PSA markers in samples containing Gleason 7 are negative may be due to tissue damage before analysis, for example, insufficient or prolonged stabilization of formalin. Immunohistochemical expression of PSA can help identify the origin of the prostate in small cell carcinomas. Analysis of more than 2,800 non-prostate tumors showed that PSA-positive staining was not entirely prostate-specific. However, cancers express various proteins extracellularly (6, 15).
The present study's results showed no statistically significant difference between staining intensity and apical staining of the PSA marker with clinicopathological features such as lymphovascular invasion and perineural arrhythmia. The comparison between the PSA marker and clinical pathological features has been examined in previous studies. The study by Chen et al. in 2008 was carried out to examine the expression of the PSA marker by immunohistochemistry in bladder cancer. Their results showed that the cytoplasmic response to PSA was observed in 6 cases (1.4%), 3 with low staining and 3 with high staining. There was a significant comparison between PSA expression and multiplicity, large tumors (3 cm), and muscle invasion (Pt2) (9).
Overall, the results of the present study indicated that as the Gleason grade increases, the apical marker's positive rate and the PSA marker's staining intensity decrease, which is statistically significant. The results of the present study showed that PSA expression could be used as a prognostic parameter in the diagnosis of prostate cancer. It is also suggested that in future studies with a larger sample size, the comparison between apical marker expression and PSA marker staining intensity will be assessed with clinicopathological factors, survival and mortality rates, treatment response, etc.
4.1. Conclusions
The results showed that the degree of positivity of the apical marker and the intensity of PSA marker staining had a significant comparison with the Gleason score and serum level of PSA, while it had no significant comparison with age, lymphovascular and perineural invasion. It is suggested that in future studies, the expression of apical markers and the intensity of PSA marker staining in benign prostate and other cancer tissue samples be evaluated and compared.