1. Background
Functional abdominal pain disorders (FAPDs) are common problems in children and adolescents and can cause great concern to their parents (1, 2). FAPDs include functional abdominal pain not otherwise specified (FAP-NOS), irritable bowel syndrome (IBS), abdominal migraine, and functional dyspepsia (FD) so that functional abdominal pain (FAP) is the most common type (1, 2). The FAP prevalence is between 0.3% and 19% in Western children (3). FAP is found in 10 - 15% of adolescents and children. Nevertheless, a higher prevalence has been reported in some studies (4).
Although the diagnosis of FAP is made when there are no organic and infectious reasons, structural and biochemical processes, disorders, etc., it is a major challenge for the family and pediatric gastroenterologist because first, it is a common disorder in children and second, there are no definite etiology and effective management to control and decrease symptoms. Therefore, it is necessary to deeply change school, sports, and daily activities (5). It is a debilitating disorder (6, 7). A national study on 20000 adolescents revealed that the risk of depression among FAP children was 45%. They had fewer physical activities and suffered from feelings of tiredness, sadness, and loneliness (8).
The exact cause of FAP is unknown, but dysmotility is one of its possible causes (1-3). Domperidone (a prokinetic agent) with antidopaminergic effects leads to increasing gastrointestinal (GI) motility. Often, it is used for abdominal discomfort, bloating, constipation, heartburn, nausea, and vomiting. Domperidone enhances antroduodenal contractions and peristalsis and thereby accelerates the transit of stomach contents (9, 10). In one study, a 7-day treatment with domperidone compared to the placebo improved gastric emptying (11).
2. Objectives
Since few studies have been performed on the use of prokinetic agents, such as domperidone, in FAP children, the aim of this study was to evaluate the effect of domperidone compared to the placebo in the treatment of FAP children.
3. Methods
Study design and participants: This double-blind, randomized, placebo-controlled clinical trial was conducted for one year (from December 2020 to December 2021) in a Pediatric Gastroenterology Clinic at Amirkola Children’s Hospital in Babol (North of Iran). The study was blinded to patients and investigators. Eligible participants were 5 - 14-year-old children who met the criteria of the Rome IV diagnostic (at least four times in a month or two months before diagnosis) for FAP (Box 1) (12). The exclusion criteria were children with chronic and underlying disease, children taking antibiotics, probiotics, and prebiotics from one month prior to the study, as well as children with alarm symptoms and signs, like pain leading to the child waking up from sleep, persistent right lower quadrant (RLQ) and right upper quadrant (RUQ) pain, dysphagia, severe vomiting (periodic, recurrent, biliary, or worrisome for a physician), nocturnal or severe chronic diarrhea, unexplained fever, unwanted weight loss, genitourinary symptoms, descending trend in the growth curve, GI bleeding, delayed puberty, back pain, localized fullness or mass, localized tenderness in RUQ or RLQ, splenomegaly, arthritis, jaundice, hepatomegaly, costovertebral angle tenderness, perianal area disease, positive family history of celiac and peptic ulcer disease, vertebral tenderness, hematochezia and anemia, abnormal or unexplained findings in the examination, chronic constipation, and inflammatory bowel disease.
| Diagnostic criteria must be fulfilled at least four times in a month or two months before diagnosis and include all of the following: |
|---|
| 1. Episodic or continuous abdominal pain that does not occur solely during physiologic events (e.g., eating and menses). |
| 2. Insufficient criteria for irritable bowel syndrome, functional dyspepsia, or abdominal migraine. |
| 3. After appropriate evaluation, the abdominal pain cannot be fully explained by another medical condition. |
a With permission from the Rome Foundation (12).
The study was approved by the Ethics Committee of Babol University of Medical Sciences (http://ethics.research.ac.ir/IR.MUBABOL.REC.1399.320), and the Iranian Registry of Clinical Trials (IRCT20160308026973N2) preregistered it. The parents of all selected children signed the informed consent form.
3.1. Intervention
The eligible children were first completely examined by a pediatric gastroenterologist and then evaluated by a pediatric cardiologist in order to assess arrhythmia and underlying heart disease. After the initial examination, the FAP children were assigned to two groups using the simple randomization method (random numbers generated by computer). Group 1 was given domperidone, and group 2 received the placebo. Before the intervention, basic information was recorded by a blinded pediatric resident, which included demographic data and pain characteristics. The severity of pain was evaluated using the Wong-Baker FACES® Pain Rating Scale, including six faces representing the pain effect. This scale ranges from a relaxed face on the left (score 0 for no hurt) to a face suggesting intense pain (score 10 for worse hurts) on the right. The child was asked to choose at the time of pain which face s/he has, then that face's score was assigned to him/her (13). The pain duration and frequency were delineated by the number of pain episodes per day (based on minutes in a day) and per month (based on days in a month), respectively.
The intervention in group 1 included a domperidone tablet of 0.25 mg/kg three times a day for eight consecutive weeks. The second group received placebo tablets in the same order. The placebo was completely similar to domperidone in terms of shape, appearance, and color, and both of them were delivered to patients in similar coded packages. Domperidone and placebo were manufactured by Hakim Company (Tehran, Iran) and the Faculty of Pharmacy, Sari University of Medical Sciences, respectively.
In addition, children and their parents were advised to avoid foods such as carbonated drinks, preservatives, and fast food and to eat right. During the current study, if children had side effects, including headache, dry mouth, dizziness, irritability, muscle cramps, sleep problems, and chest pain, their parents were asked to fill checklist of side effects and call the pediatric resident to stop the medicine if necessary. In addition, patients were followed up by telephone every week in terms of consumption control and occurrence of symptoms by the blinded pediatric assistant. At the end of the study, the duration, intensity, and frequency of pain were assessed by the same pediatric resident under the supervision of a pediatric gastroenterologist.
3.2. Outcome Measures and Follow-Up
The primary outcome indicated that the recovery rate was at least a 50% reduction in both frequency and severity of pain, and the secondary outcome was a significant reduction in the pain duration, frequency, and intensity compared to baseline.
3.3. Sample Size and Test Power
According to a previous study (14), the reduction in the severity of abdominal pain was 54.1% and 24.7% in intervention and placebo groups at eight weeks, respectively. Regarding these data and the confidence of 95% and test power of 80%, the adequate sample size was calculated 50 participants in each group. Assuming a drop-out of 5%, 105 children were estimated.
3.4. Statistical Analysis
The data were analyzed using SPSS 16 (Inc., Chicago, IL, USA). The results of the present study were compared using the protocols. Data were expressed as numbers (percentage) or mean ± SD. Before analysis, the Kolmogorov-Smirnov test for normal distribution was performed. The chi-square test and independent t-test performed comparisons between groups. In addition, equivalent nonparametric tests were used when necessary. Statistically, a two-sided P < 0.05 was set as a significant level in all analyses.
4. Results
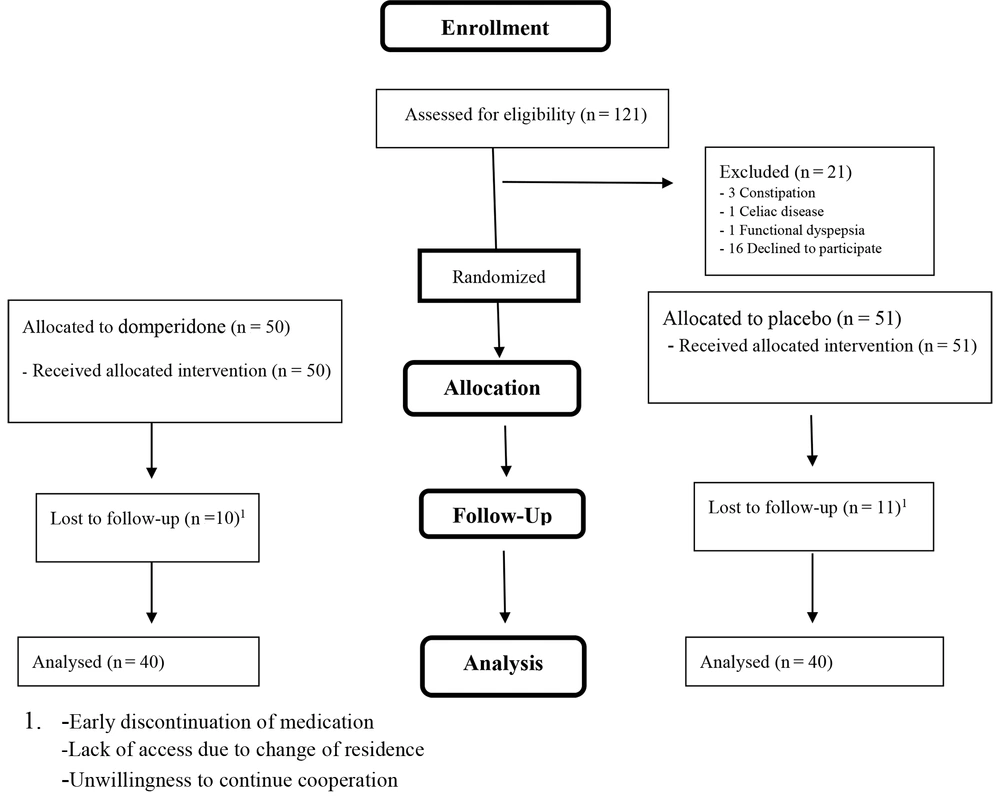
Totally, 80 children (each group = 40 children) completed the treatment period, and 45 (56.3%) of them were females (Figure 1). The mean age and weight of the total participants were 8.64 ± 2.87 years and 29.49 ± 12.03 Kg, respectively. There were no significant differences among the subjects in terms of baseline characteristics (Table 1).
| Variables | Domperidone | Placebo | P-Value |
|---|---|---|---|
| Age (y) | 8.53 ± 2.75 | 8.75 ± 3.04 | 0.7 |
| Male/female (No.) | 18/22 | 17/23 | 0.82 |
| Weight (kg) | 29.08 ± 12.29 | 29.9 ± 11.90 | 0.76 |
| Frequency of pain (No./week) | 11.15 ± 6.03 | 16.33 ± 11.00 | 0.13 |
| Intensity of pain | 6.35 ± 0.89 | 6.10 ± 1.49 | 0.46 |
| Duration of pain (min/day) | 37.53 ± 45.08 | 35.70 ± 44.90 | 0.84 |
a Values are expressed as mean ± SD unless otherwise indicated.
4.1. Primary and Secondary Outcome Measures
The overall recovery rate was 48.74% (38.75% - 60.00%), and it was 71.8% (55.26% - 84.62%) and 28.2% (13.95% - 42.85%) in the domperidone and placebo groups, respectively. There was a significant difference between the two groups (P < 0.0001).
The comparisons between the two groups in terms of intensity, duration, and frequency of pain are presented in Table 2, indicating that there was a significant difference between the two groups after the intervention. The comparison within groups indicated a significant reduction in the intensity (P < 0.0001), duration (P < 0.0001), and frequency (P < 0.0001) of pain in both the intervention and control groups. There were no adverse events in both group during the study period.
| Variables | Domperidone | Placebo | P-Value |
|---|---|---|---|
| Frequency of pain (No./week) | 3.35 ± 3.99 | 10.63 ± 10.55 | < 0.001 |
| Intensity of pain | 2.20 ± 2.16 | 5.05 ± 2.37 | < 0.001 |
| Duration of pain (min/day) | 4.58 ± 7.71 | 24.50 ± 41.45 | < 0.001 |
a Values are expressed as mean ± SD.
5. Discussion
This prospective randomized study suggested that domperidone than placebo could provide a better outcome to control the pain in FAP children. In the ongoing study, after 8-week treatment, there was a significant reduction in severity, duration, and frequency of pain. Domperidone used to treat upper GI motility disorders, is a dopamine antagonist with prokinetic properties (10). Prokinetic drugs are safe and reduce abdominal symptoms in children (15-17).
There are few placebo-controlled trials on the therapeutic effects of domperidone on abdominal pain in children, and more studies have been performed on adults with abdominal pain-predominant functional GI disorders (AP-FGIDs) (18-21). In the present study, the recovery rate showed at least a 50% reduction in both frequency and severity of pain in the intervention group, which was more than twice as high as that in the placebo group (71.8% vs. 28.2%). Further, at the end of the eighth week, neither side effects nor adverse reactions were observed in both groups.
In the study by Karunanayake et al. (22), administration of domperidone or placebo for eight weeks in children with AP-FGIDs caused a recovery rate of 44% and 28% in the domperidone and placebo groups, respectively, and this difference was not significant between the two groups. At the 6-month follow-up, it was 50% and 38% in the domperidone and placebo groups, respectively, and this difference was statistically significant. Compared to the ongoing study, the mentioned study evaluated FD and IBS children in addition to FAP children and represented that domperidone had a therapeutic effect on the treatment of FAP children but had no therapeutic effect on FD and IBS children both at eight weeks and six months, which is consistent with the results of the present study, in which as a secondary outcome, the pain intensity decreased in the domperidone group than in placebo group after eight weeks.
Taghvaei et al. (14) found that the quality of life score was more in the adult patients treated with domperidone and pantoprazole than in the control group (placebo and pantoprazole). Davis et al. (20) studied 18-48-year-old patients with chronic unexplained upper GI pain and concluded that symptoms significantly reduced in the domperidone group compared to the control group after two weeks.
A comparative study evaluated the effectiveness of prokinetic drugs and laxatives in 4 - 15-year-old children who met the Rome III criteria for FAP and occult constipation and illustrated that laxatives were more effective than domperidone in decreasing abdominal pain in children with occult constipation (23).
In a study conducted by Hamidian (21), the effects of metoclopramide and domperidone were compared in FD patients, and there was no significant difference in therapeutic response between the two groups, but side effects were significantly lower in the domperidone group.
The main mechanism for relieving the pain after domperidone administration is unknown, and it is improbable that this effect is related to the prokinetic properties of the drug. Domperidone may act in a different way to modulate pain. Although dopamine is a neurotransmitter in the brain that fights pain, domperidone is unlikely to modulate pain in central pain centers because it does not cross the blood-brain barrier (24, 25). More studies are needed to demonstrate the main mechanism of pain relief in children treated with domperidone.
A homogeneous group of children with FAP was evaluated in the current study. This can be considered the main strength of the study. The current study had several limitations. First, a self-reported face pain scale as a subjective tool was applied to determine the intensity of abdominal pain. However, it has been used commonly in other studies and is considered a valid scale in children. Second, the comparison within the placebo group was significant unexpectedly; hence, a larger sample size was required. The third one was the lack of long-term follow-up of patients to better assess the useful effects of domperidone on the FAP treatment and the lack of antroduodenal manometry or nuclear scan of gastric emptying to better evaluate the effectiveness of domperidone. Finally, there was not enough study on children to compare the results more accurately; thus, it is recommended to do further studies with longer follow-ups and larger sample sizes along with paraclinical studies to better assess the effectiveness of domperidone in FAP children. According to the Rome committee recommendation, at least a 6-month follow-up is required to establish the long-term efficacy of the treatment for FGIDs (12).
5.1. Conclusion
The used domperidone significantly decreased the duration, frequency, and severity of abdominal pain in FAP children during an 8-week trial without side effects.