1. Background
In March 2020, the World Health Organization (WHO) declared the outbreak of COVID-19 a serious global public health problem (1-4). At the early stages of the disease, the inability to predict the severity of the infection leads to increased mortality rates (5). So far, COVID-19 has infected more than 230 million cases and caused more than 4.7 million deaths (6). Previous studies have shown that diabetes and cardiovascular disease increase the incidence of Severe Acute Respiratory Syndrome (SARS) and Middle East Respiratory Syndrome (MERS), which are acute respiratory syndromes similar to COVID-19. More than 50% of people with MERS had diabetes and hypertension, and more than 30% had an underlying cardiovascular disease (7). Diabetes and cardiovascular disease increase the risk of SARS-related deaths by 11% and 8%, respectively. Chest computed tomography (CT) imaging plays a valuable role in detecting the severity and progress of COVID-19 pneumonia (8-12). COVID-19-positive patients have specific CT imaging features of multiple ground-glass opacity (GGO), which reflect the severity of pulmonary inflammation. Chest CT features can be considered a noteworthy tool for the prognostic prediction and treatment strategy of patients with COVID-19; these features are associated with disease severity (13).
In summary, underlying conditions such as hypertension, cardiovascular disorders, and diabetes can increase COVID-19 severity and affect symptoms, diagnosis, treatment, and prognosis; hence, COVID-19-related mortality also increases in these patients (14). Prioritizing medical resources in clinics should be based on the severity of COVID-19, especially when resources and staff are limited.
2. Objectives
The current study investigated the prognostic factors associated with demographic parameters, clinical and vital signs, and laboratory results for predicting severity and mortality in patients infected with COVID-19.
3. Methods
3.1. Patient Selection
This retrospective analysis was conducted from Sep 2020 to Sep 2021 on the medical records of 372 COVID-19-positive patients (199 male and 173 female) who were admitted to Khatam Al-Anbia Hospital. Regarding the research objectives, in a previous study (15), the mortality ratio in confirmed COVID-19 patients (P = 0.135), α = 0.05, d = 0.04, and using the sample size ratio formula, the minimum sample size was estimated as 280. The subjects were enrolled in the study via a purposive sampling method. Nasopharyngeal swabs were utilized to diagnose COVID-19 by Real-time RT–PCR. Cases under 15 years of age were not included in the study. The 372 patients were divided into the non-severe group (n = 275) and the severe group (n = 97). A checklist was utilized to record patients' data. All the data was kept confidential in compliance with the principles of the Declaration of Helsinki; the requirement for informed consent was waived. This study was approved by the research ethics committee of our institute (ethics code: IR.SHOUSHTAR.REC.1399.006); (webpage of ethical approval code: ethics.research.ac.ir/ProposalCertificateEn.php?id=131480).
3.2. Data Collection
Data collection was done at the beginning of the hospitalization. Data included demographic information (age, gender, marital status, etc.), clinical information and vital signs (underlying conditions, fever, cough, dyspnea, etc.), and paraclinical information (laboratory results). The laboratory results included fasting blood sugar (FBS), blood urea nitrogen (BUN), creatinine (Cr), sodium (Na), potassium (K), white blood cells (WBC), red blood cells (RBC), hemoglobin (Hb), hematocrit (HCT), platelet (PLT), lymphocyte (Lym), neutrophils (Neut), arterial blood gas test (PH, pO2, pCO2, HCO3), erythrocyte sedimentation rate (ESR), prothrombin time (PT), partial thromboplastin time (PTT), international normalized ratio (INR), and oxygen saturation (SpO2). Survival/death outcomes were also collected.
3.3. Chest CT Acquisition and Image Analysis
All chest CT images were acquired with a multi-slice CT scanner (GE Healthcare, USA). Patients were scanned in a supine position during breath-holding; they were trained to hold their breath at the tidal inspiration level during the scan. Imaging parameters were as follows: kVP = 120, mAs = 115, matrix size = 512 × 512, slice thickness = 7.5 mm. COVID-19 disease severity was determined based on the severity of pulmonary involvement using CT chest images. All CT images were subjectively assessed by an emergency medicine specialist, blinded to clinical and laboratory results. The severity of lung involvement was visually scored using the CT severity score. Each of the five lung lobes was assessed for percentage of involvement and classified as follows: 5% or less = 1, 5% - 25% = 2, 26% - 49% = 3, 50% - 75% = 4, > 75% = 5. An overall lung “total severity score” was reached by summing the five lobe scores (total score out of 25). Severity was then categorized into non-severe (score < 18) and severe (score ≥ 18) (16-18).
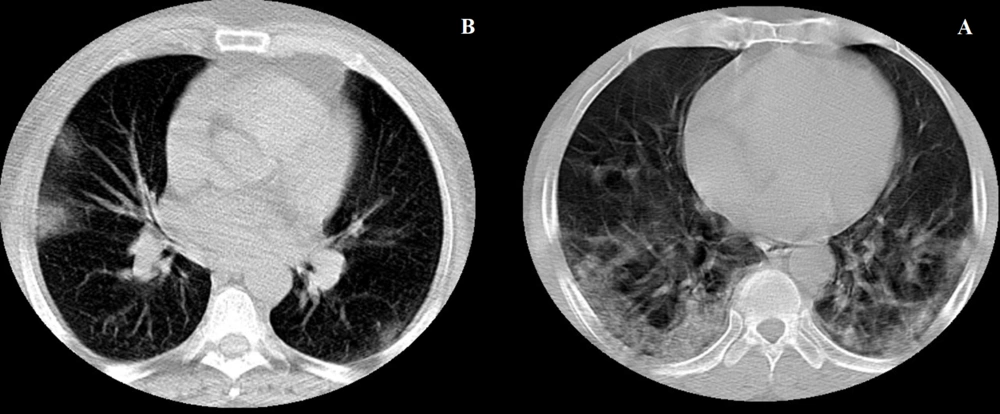
The main features of CT images in COVID-19 pneumonia are described as peripheral, bilateral, and basal predominant ground-glass opacities (GGO) and/or consolidation with superimposed irregular lines and interfaces (19) (Figure 1).
CT manifestation in patients with COVID-19 pneumonia; (A) Axial chest CT scan in a 43-year-old woman shows bilateral areas of GGO in mixed peripheral and central distributions (non-severe COVID-19); (B) Axial chest CT scan in a 63-year-old man shows bilateral areas of GGO, consolidation, and crazy-paving sign in a mixed peripheral and central distribution (severe COVID-19).
3.4. Statistical Analysis
Patients' characteristics (demographic and clinical data) and laboratory results (paraclinical data) were recorded on the specific checklist designed for this study.
The collected data were analyzed using IBM SPSS software for Windows (version 18). Descriptive statistics for the quantitative variables were presented as mean, standard deviation, and median (IQR: Q1-Q3), and qualitative (categorical) variables were reported as the frequency with percentage. Independent t-test or Mann-Whitney test was employed to compare means, ANOVA or Kruskal-Wallis test to compare means between levels of variables, and chi-square test and odds ratio to compare qualitative variables. Selecting a statistical test depends on data normality, which was assessed using the Kolmogorov-Smirnov test. Logistic regression analysis was employed using the Forward LR method to evaluate the relationship between some demographic parameters, underlying conditions, and laboratory results to predict COVID-19 severity and mortality. In regression models, "survival" and "non-severe" were considered baseline categories. The meaningless level was considered as P < 0.05.
4. Results
In this study, 372 patients with COVID-19 were examined with a mean age of 50.22 ± 16.67. 46.5% (n = 173) of patients were female, and 53.5% (n = 199) were male. 25.3% of patients (n = 94) had no underlying conditions, and 24.7% (n = 92) had diabetes. The most common symptoms in COVID-19 patients were anosmia (74.7%) and cough (52.7%). The rates of mortality and the severe form of the disease were reported as 12.9% (n = 48) and 87.1% (n = 324), respectively. The results of comparing patients' characteristics and laboratory results according to COVID-19-related severity are shown in Table 1. Demographic parameters, including gender, marital status, and place of residence, did not affect the severity of the disease (P > 0.05). In contrast, underlying conditions like diabetes mellitus (P < 0.001), hypertension (P = 0.024), and pulmonary diseases (P = 0.038) were associated with COVID-19 severity; patients with these diseases suffered from a severe form of COVID-19. This study considered the history of infection with different types of pulmonary diseases, including airway, lung tissue, and lung circulation diseases. The risk of getting infected with severe COVID-19 in diabetic patients was 2.63 times higher than in non-diabetic cases (OR = 2.63, CI: 1.58 - 4.37, P < 0.001). The risk of getting infected with severe COVID-19 in patients with hypertension was 2.23 times higher than in patients without hypertension (OR = 2.23, CI: 1.14 - 4.35, P = 0.023). The risk of getting infected with severe COVID-19 in patients with the pulmonary disease was 2.23 times higher than in patients without the pulmonary disease (OR = 2.42, CI: 1.02 - 5.73, P = 0.038). Anosmia was inversely associated with the severity of the disease; patients with anosmia had a non-severe form of the disease (OR = 0.466, CI: 0.237 - 0.918, P = 0.043). Other symptoms and vital signs did not affect the severity of COVID-19. As presented in Table 1, FBS (P = 0.014), BUN (P = 0.045), Cr (P = 0.027), WBC (P = 0.034), and Neut (P = 0.002) levels were higher in patients with severe COVID-19. In contrast, the levels of SpO2 (P = 0.001) and pCO2 (P = 0.014) were lower in these patients.
| Patient Characteristics and Laboratory Results | Number of Patients (%) | Disease Severity | P-Value | |
|---|---|---|---|---|
| Non-severe | Severe | |||
| Age (y) | 50.22 ± 16.67 | 49.4 ± 16.58 | 52.48 ± 16.78 | 0.118 |
| Weight (kg) | 77.14 ± 15.70 | 78.11 ± 16.26 | 74.12 ± 13.51 | 0.210 |
| Height (cm) | 168.61 ± 12.75 | 166.22 ± 11.04 | 169.26 ± 17.20 | 0.522 |
| BMI (kg/m2) | 27.06 ± 5.23 | 27.23 ± 5.48 | 26.49 ± 4.30 | 0.568 |
| Gender | 0.226 | |||
| Female | 173 (46.5) | 133 (76.9) | 40 (23.1) | |
| Male | 199 (53.5) | 142 (71.4) | 57 (28.6) | |
| Place of residence | 0.072 | |||
| City | 213 (57.3) | 165 (77.5) | 48 (22.5) | |
| Village | 159 (42.7) | 110 (69.2) | 49 (30.8) | |
| Marital status | 0.898 | |||
| Married | 290 (78.0) | 216 (74.5) | 74 (25.5) | |
| Single | 46 (12.4) | 33 (71.7) | 13 (28.3) | |
| Widowed | 36 (9.7) | 26 (72.2) | 10 (27.8) | |
| Diabetes | < 0.001 b | |||
| Yes | 92 (24.7) | 37 (40.2) | 55 (59.8) | |
| No | 280 (75.3) | 220 (78.6) | 60 (21.4) | |
| Hypertension | 0.024 b | |||
| Yes | 42 (11.3) | 17 (40.5) | 25 (59.5) | |
| No | 330 (88.7) | 250 (75.8) | 80 (24.2) | |
| Cardiovascular disease | 0.805 | |||
| Yes | 29 (7.8) | 22 (75.9) | 7 (24.1) | |
| No | 343 (92.2) | 253 (73.8) | 90 (26.2) | |
| Renal disease | 0.081 | |||
| Yes | 15 (4.0) | 14 (93.3) | 1 (6.7) | |
| No | 357 (96.0) | 261 (73.1) | 96 (26.9) | |
| Pulmonary disease | 0.038 b | |||
| Yes | 23 (6.2) | 10 (43.5) | 13 (56.5) | |
| No | 349 (93.8) | 265 (75.9) | 84 (24.1) | |
| Sense of smell | 0.043 b | |||
| With anosmia | 163 (74.7) | 128 (78.5) | 35 (21.5) | |
| Without anosmia | 55 (25.3) | 36 (65.5) | 19 (34.5) | |
| Fever | 0.143 | |||
| Yes | 127 (34.1) | 88 (69.3) | 39 (30.7) | |
| No | 245 (65.9) | 187 (76.3) | 58 (23.7) | |
| Cough | 0.357 | |||
| Yes | 196 (52.7) | 141 (71.9) | 55 (28.1) | |
| No | 176 (47.3) | 134 (76.1) | 42 (23.9) | |
| Dyspnea | 0.124 | |||
| Yes | 115 (30.9) | 36 (31.3) | 79 (68.7) | |
| No | 257 (69.1) | 196 (76.3) | 61 (23.7) | |
| Muscle pain | 0.673 | |||
| Yes | 90 (24.2) | 65 (72.2) | 25 (27.8) | |
| No | 282 (75.8) | 210 (74.5) | 72 (25.5) | |
| Headache | 0.871 | |||
| Yes | 67 (18.0) | 49 (73.1) | 18 (26.9) | |
| No | 305 (82.0) | 226 (74.1) | 79 (25.9) | |
| Nausea and vomiting | 0.649 | |||
| Yes | 35 (9.4) | 27 (77.1) | 8 (22.9) | |
| No | 337 (90.6) | 248 (73.6) | 89 (26.4) | |
| Sore throat | 0.151 | |||
| Yes | 46 (12.4) | 30 (65.2) | 16 (34.8) | |
| No | 326 (87.6) | 245 (75.2) | 81 (24.8) | |
| Anorexia | 0.472 | |||
| Yes | 75 (20.2) | 53 (70.7) | 22 (29.3) | |
| No | 297 (79.8) | 222 (74.7) | 75 (25.3) | |
| Anosmia recovery (day) | 5.21 ± 8.42 | 5.24 ± 7.69 | 5.20 ± 10.44 | 0.170 |
| FBS (mg/dL) | 179.0 ± 95.13 | 167.25 ± 91.39 | 200.92 ± 99.81 | 0.014 b |
| BUN (mg/dL) | 17.89 ± 13.73 | 16.26 ± 11.45 | 20.76 ± 16.72 | 0.045 b |
| Cr (mg/dL) | 1.05 ± 0.605 | 0.89 ± 0.62 | 1.17 ± 0.55 | 0.027 b |
| NA (mmol/L) | 140.54 ± 16.86 | 139.57 ± 3.75 | 142.18 ± 18.20 | 0.656 |
| K (mmol/L) | 4.26 ± 0.53 | 4.22 ± .45 | 4.32 ± 0.64 | 0.381 |
| WBC (109/L) | 6.99 ± 3.73 | 6.46 ± 2.91 | 8.28 ± 4.79 | 0.034 b |
| RBC (1012/L) | 4.73 ± 2.36 | 4.83 ± 2.89 | 4.55 ± .70 | 0.672 |
| Hb (g/dL) | 12.34 ± 1.80 | 12.36 ± 1.67 | 12.30 ± 2.04 | 0.736 |
| HCT (%) | 39.10 ± 25.36 | 39.85 ± 31.20 | 37.70 ± 5.48 | 0.720 |
| PLT (109/L) | 223.07 ± 110.47 | 224.79 ± 120.42 | 219.90 ± 89.94 | 0.873 |
| Lym (109/L) | 32.38 ± 26.19 | 33.78 ± 27.41 | 29.67 ± 23.58 | 0.192 |
| Neut (109/L) | 64.17 ± 15.66 | 61.90 ± 16.64 | 69.56 ± 12.52 | 0.002 b |
| MXD | 9.26 ± 7.10 | 9.91 ± 8.02 | 7.90 ± 4.38 | 0.075 |
| PH | 7.31 ± 0.587 | 7.17 ± 1.15 | 7.35 ± 0.07 | 0.112 |
| pCO2 (mmHg) | 36.86 ± 5.92 | 37.85 ± 5.99 | 33.61 ± 4.41 | 0.001 b |
| pO2 (mmHg) | 36.24 ± 16.55 | 41.74 ± 16.81 | 36.87 ± 16.31 | 0.075 |
| HCO3 (meq/L) | 21.84 ± 3.27 | 21.24 ± 2.89 | 21.02 ± 3.37 | 0.272 |
| ESR (mm/h) | 32.25 ± 23.22 | 31.07 ± 25.85 | 33.74 ± 19.97 | 0.226 |
| PT (s) | 20.06 ± 17.12 | 21.63 ± 19.50 | 15.89 ± 7.20 | 0.642 |
| PTT (s) | 42.07 ± 30.63 | 35.74 ± 18.13 | 55.44 ± 46.15 | 0.445 |
| INR | 1.10 ± 0.17 | 1.11 ± 0.13 | 1.09 ± 0.24 | 0.940 |
| SpO2 | 60.73 ± 20.98 | 87.25 ± 6.2 | 57.54 ± 19.81 | 0.014 b |
a Values are expressed as No. (%) or mean ± SD.
b Significance level: P < 0.05.
The results of comparing patients' characteristics and laboratory results according to COVID-19-related mortality are shown in Table 2. Demographic parameters, including gender, marital status, and place of residence, did not affect the mortality rate (P > 0.05). In contrast, underlying conditions like diabetes mellitus (P = 019), hypertension (P = 0.012), cardiovascular (P = 0.037), and pulmonary (P < 0.001) diseases were effective in COVID-19-related mortality. This study considered the history of cardiovascular diseases, including coronary heart disease, strokes and transient ischemic attacks, and peripheral arterial and aortic diseases. The risk of COVID-19-related death in diabetic patients was 2.53 times higher than in non-diabetic cases (OR = 2.53, CI: 1.19 - 4.24, P = 0.019). The risk of COVID-19-related death in patients with hypertension was 2.81 times higher than in patients without hypertension (OR = 2.23, CI: 1.30 - 6.05, P = 0.012). The risk of COVID-19-related death in patients with cardiovascular disease was 2.88 times higher than in patients without cardiovascular disease (OR = 2.88, CI: 1.19 - 6.94, P = 0.037). The risk of COVID-19-related death in patients with pulmonary disease was 7.73 times higher than in patients without the pulmonary disease (OR = 7.73, CI: 3.18 - 18.75, P < 0.001). Anosmia was inversely associated with COVID-19-related mortality. The risk of COVID-19-related death was lower in patients with anosmia (OR = 0.413, CI: 0.172 - 0.995, P = 0.044). In contrast, other symptoms, including fever (OR = 2.36, CI: 1.28 - 4.36, P = 0.008) and dyspnea (OR = 2.10, CI: 1.13 - 3.89, P = 0.02), were directly associated with COVID-19-related mortality. Patients who died of COVID-19 (59.62 ± 15.27) were older than those who recovered from it (48.80 ± 16.43) (P < 0.001). As presented in Table 1, FBS (P = 0.045), BUN (P < 0.001), Cr (P = 0.047), and Neut (P = 0.005) levels of patients who died of COVID-19 were higher than in recovered patients. SpO2 levels of patients who died of COVID-19 were lower than recovered patients (P < 0.001).
| Patient Characteristics and Laboratory Results | Number of Patients (%) | Death | P-Value | |
|---|---|---|---|---|
| Yes | No | |||
| Age (y) | 50.22 ± 16.67 | 48.80 ± 16.43 | 59.62 ± 15.27 | < 0.001 b |
| Weight (kg) | 77.14 ± 15.70 | 77.81 ± 15.5 | 72.17 ± 16.20 | 0.361 |
| Height (cm) | 168.61 ± 12.75 | 169.45 ± 10.81 | 162.32 ± 21.92 | 0.193 |
| BMI (kg/m2) | 27.06 ± 5.23 | 27.08 ± 5.20 | 26.91 ± 5.53 | 0.887 |
| Gender | 0.280 | |||
| Female | 173 (46.5) | 177 (88.9) | 22 (11.1) | |
| Male | 199 (53.5) | 147 (85.0) | 26 (15.0) | |
| Place of residence | 0.279 | |||
| City | 213 (57.3) | 135 (84.9) | 24 (15.1) | |
| Village | 159 (42.7) | 189 (88.7) | 24 (11.3) | |
| Marital status | 0.660 | |||
| Married | 290 (78.0) | 251 (86.6) | 39 (13.4) | |
| Single | 46 (12.4) | 42 (91.3) | 4 (8.7) | |
| Widowed | 36 (9.7) | 31 (86.1) | 5 (13.9) | |
| Diabetes | 0.019 b | |||
| Yes | 92 (24.7) | 73 (79.3) | 19 (20.7) | |
| No | 280 (75.3) | 251 (89.6) | 29 (10.4) | |
| Hypertension | 0.012 b | |||
| Yes | 42 (11.3) | 31 (73.8) | 11 (26.2) | |
| No | 330 (88.7) | 293 (88.8) | 37 (11.2) | |
| Cardiovascular disease | 0.037 b | |||
| Yes | 29 (7.8) | 21 (72.4) | 8 (27.6) | |
| No | 343 (92.2) | 303 (88.3) | 40 (11.7) | |
| Renal disease | 0.081 | |||
| Yes | 15 (4.0) | 14 (93.3) | 1 (6.7) | |
| No | 357 (96.0) | 310 (86.8) | 47 (13.2) | |
| Pulmonary disease | < 0.001 b | |||
| Yes | 23 (6.2) | 12 (52.2) | 11 (47.8) | |
| No | 349 (93.8) | 312 (89.4) | 37 (10.6) | |
| Sense of smell | 0.044 b | |||
| With anosmia | 163 (74.7) | 149 (91.4) | 14 (8.6) | |
| Without anosmia | 54 (25.3) | 44 (81.8) | 10 (18.2) | |
| Fever | 0.008 b | |||
| Yes | 127 (34.1) | 102 (80.3) | 25 (19.7) | |
| No | 245 (65.9) | 222 (90.6) | 23 (9.4) | |
| Cough | 0.537 | |||
| Yes | 196 (52.7) | 173 (88.3) | 23 (11.7) | |
| No | 176 (47.3) | 151 (85.8) | 25 (14.2) | |
| Dyspnea | 0.020 b | |||
| Yes | 115 (30.9) | 93 (80.9) | 22 (19.1) | |
| No | 257 (59.1) | 231 (89.9) | 26 (10.1) | |
| Muscle pain | 0.718 | |||
| Yes | 90 (24.2) | 80 (88.9) | 10 (11.1) | |
| No | 282 (75.8) | 244 (86.5) | 38 (13.5) | |
| Headache | 0.795 | |||
| Yes | 67 (18.0) | 59 (88.1) | 8 (11.9) | |
| No | 305 (82.0) | 265 (86.9) | 40 (13.1) | |
| Nausea and vomiting | 0.287 | |||
| Yes | 35 (9.4) | 33 (94.3) | 2 (5.7) | |
| No | 337 (90.6) | 291 (86.4) | 46 (13.4) | |
| Sore throat | 0.483 | |||
| Yes | 46 (12.4) | 42 (91.3) | 4 (8.7) | |
| No | 326 (87.6) | 282 (86.5) | 44 (13.5) | |
| Anorexia | 0.342 | |||
| Yes | 75 (20.2) | 68 (90.7) | 7 (9.3) | |
| No | 297 (79.8) | 256 (86.2) | 41 (13.8) | |
| Anosmia recovery (day) | 5.21 ± 8.42 | 5.04 ± 7.59 | 6.58 ± 13.53 | 0.320 |
| FBS (mg/dL) | 179.0 ± 95.13 | 172.86 ± 92.73 | 211.00 ± 102.21 | 0.045 b |
| BUN (mg/dL) | 17.89 ± 13.73 | 16.32 ± 11.29 | 25.55 ± 20.61 | 0.001 b |
| Cr (mg/dL) | 1.05 ± 0.605 | 1.03 ± 0.61 | 1.17 ± 0.55 | 0.047 b |
| NA (mmol/L) | 140.54 ± 16.86 | 140.12 ± 3.99 | 138.75 ± 4.19 | 0.128 |
| K (mmol/L) | 4.26 ± 0.53 | 4.22 ± 0.49 | 4.44 ± 0.69 | 0.108 |
| WBC (109/L) | 6.99 ± 3.73 | 6.76 ± 3.04 | 8.28 ± 6.26 | 0.845 |
| RBC (1012/L) | 4.73 ± 2.36 | 4.77 ± 2.55 | 4.56 ± 0.82 | 0.826 |
| Hb (g/dL) | 12.34 ± 1.80 | 12.39 ± 1.76 | 12.03 ± 2.07 | 0.443 |
| HCT (%) | 39.10 ± 25.36 | 39.41 ± 27.45 | 37.38 ± 5.45 | 0.833 |
| PLT (109/L) | 223.07 ± 110.47 | 225.02 ± 115.70 | 212.16 ± 75.03 | 0.721 |
| Lym (109/L) | 32.38 ± 26.19 | 33.64 ± 16.85 | 27.79 ± 11.75 | 0.064 |
| Neut (109/L) | 64.17 ± 15.66 | 63.08 ± 15.87 | 71.77 ± 12.52 | 0.005 b |
| MXD | 9.26 ± 7.10 | 9.47 ± 7.22 | 6.86 ± 5.10 | 0.086 |
| PH | 7.31 ± 0.587 | 7.28 ± 0.71 | 7.35 ± 0.08 | 0.923 |
| pCO2 (mmHg) | 36.86 ± 5.92 | 37.04 ± 5.42 | 36.53 ± 6.83 | 0.833 |
| pO2 (mmHg) | 36.24 ± 16.55 | 35.20 ± 13.82 | 38.21 ± 20.79 | 0.642 |
| HCO3 (meq/L) | 21.84 ± 3.27 | 22.12 ± 2.64 | 21.31 ± 4.20 | 0.550 |
| ESR (mm/h) | 32.25 ± 23.22 | 29.80 ± 20.18 | 42.20 ± 31.79 | 0.064 |
| PT (s) | 20.06 ± 17.12 | 21.20 ± 19.20 | 16.50 ± 7.55 | 0.843 |
| PTT (s) | 42.07 ± 30.63 | 32.00 ± 7.27 | 67.25 ± 6.15 | 0.147 |
| INR | 1.10 ± 0.17 | 1.10 ± 0.12 | 1.11 ± 0.27 | 0.639 |
| SpO2 | 60.73 ± 20.98 | 31.46 ± 9.02 | 67.09 ± 16.98 | 0.001 b |
a Values are expressed as No. (%) or mean ± SD.
b Significance level: P < 0.05.
Tables 3 and 4 summarize multiple logistic regression analysis results. As presented in Table 4, the variables of the place of residence, diabetes, PCO2, and BUN affect the severity of the disease. Diabetes disease, living in the village, higher PCO2, and higher BUN levels were more likely to develop the risk of disease severity. As shown in Table 4, age, pulmonary diseases, and BUN variables affect mortality rates. Increasing age, pulmonary diseases, and higher BUN levels were more likely to develop the risk of death.
| Variables | Coefficients (β) | OR | 95% CI for OR | P-Value | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Place of residence | 0.514 | 1.672 | 1.007 | 2.750 | 0.044 |
| Diabetes | 0.925 | 2.524 | 1.428 | 4.220 | 0.001 |
| PCO2 | 0.164 | 1.178 | 1.028 | 1.005 | < 0.001 |
| BUN | 0.027 | 1.027 | 1.005 | 1.050 | 0.015 |
| Variables | Coefficients (β) | OR | 95% CI for OR | P-Value | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Age | 0.033 | 1.034 | 1.013 | 1.055 | 0.001 |
| Pulmonary disease | 1.827 | 6.217 | 2.392 | 16.162 | < 0.001 |
| BUN | 0.026 | 1.026 | 1.002 | 1.052 | 0.035 |
5. Discussion
The availability and validity of early predictors of disease severity is an unavoidable need in COVID-19 patients. The results from the current study found a prognostic value in predicting COVID-19 severity and mortality for some clinical (diabetes, hypertension, and pulmonary diseases) and paraclinical (FBS, BUN, Cr, Neut, and SpO2) parameters. As well cardiovascular diseases, WBC and pCO2 were also effective in predicting disease-related mortality. In this regard, the multiple logistic regression analysis results showed that the place of residence, diabetes, PCO2, and BUN affect the severity of the disease. This is while age, pulmonary diseases, and BUN affect the patients' outcomes (death/recovery). According to the results, about 60% of diabetic patients experienced severe COVID-19. In this study, the risk of getting infected with severe forms of disease and COVID-19-related death was 2.63 and 2.53 times higher in diabetic patients than in non-diabetic cases. In this regard, the results of a meta-analysis by de Almeida-Pititto et al. showed that the risk of getting infected with severe forms of COVID-19 and the mortality rate is 2.35 and 2.50 times higher in diabetic patients, respectively (20). In a meta-analysis by Kumar et al., the risk of infection with severe forms of COVID-19 and mortality rate were 2.75 and 1.90 times higher in diabetic patients than in nondiabetic individuals, respectively (21), which is consistent with the results of the present study. Nevertheless, in a meta-analysis by Pinto and Bertoluci, the risk of infection with severe forms of the disease was 3.53 times higher in diabetic patients than in non-diabetics, which is not consistent with the results of the present study. In a study by Pinto and Bertoluci, this inconsistency in the results is attributed to the influence of other factors, such as the effect of a patient's age on disease severity (22). Studies have shown that the innate immune system is inflamed due to chronic hyperglycemia (diabetes mellitus), characterized by an inappropriate cytokine response and excessive coagulation in these patients (20).
This study found that patients with a history of hypertension experienced severe forms of COVID-19. The risk of severe COVID-19 and mortality rates were 2.23 and 2.81 times higher in patients with hypertension than in those without it, respectively. In a meta-analysis by de Almeida-Pititto et al., the risk of getting infected with severe forms of COVID-19 and mortality rates was 2.88 and 2.99 times higher in patients with hypertension than in those without it (20). In a meta-analysis by Pranata et al, the risk of severe forms of COVID-19 and mortality rates was 2.04 and 2.21 times higher in patients with hypertension than in those without it (23), which is consistent with the results of our study. However, in a meta-analysis by Liu et al., the risk of severe forms of COVID-19 was 2.84 times higher in hypertensive patients, but no significant relationship was found between hypertension and the risk of death due to COVID-19. It is possible that this discrepancy is due to the fact that only three studies were reviewed in the mentioned meta-analysis; thus, its results cannot be trusted (24).
In the present study, the risk of getting infected with severe forms of COVID-19 was 2.23 times higher in patients with a history of pulmonary diseases (airway diseases, lung tissue diseases, and lung circulation diseases), and the risk of mortality was 7.73 times higher in these patients than in cases without pulmonary problems. In the study by Du et al., the risk of severe COVID-19 and mortality rates were 5.67 and 3.72 times higher, respectively (25). Numerous factors, such as smoking, respiratory problems, and lung cancer, can affect COVID-19 severity and mortality. However, in the meta-analysis by Du et al., no significant association was found between asthma and COVID-19-related mortality, which is not consistent with the present study (25).
In the current study, the risk of death due to COVID-19 in cardiovascular patients was 2.88 times higher than in patients without it. In this regard, the meta-analysis by de Almeida-Pititto et al. and the study by Mubarik et al. showed that the risk of death in patients with cardiovascular diseases was 6.34 and 3 times higher than in patients without a history of heart disease (20, 26). However, in the study by Rastad et al., there was no significant relationship between COVID-19 and increasing mortality rates in cardiovascular patients, which is basically due to the difference in the age factor; age is a risk factor for COVID-19-related mortality in most patients with cardiovascular diseases (27). The complex mechanism associated with atherosclerotic cardiovascular disease involves a pathological process associated with oxidative stress, inflammation, and a prothrombotic situation. The same mechanisms that cause vascular damage and regeneration are seen in people with type 2 diabetes, obesity, and high blood pressure (20).
Among the COVID-19 symptoms, anosmia was inversely associated with the severity and mortality rates of the disease. Severe infection and mortality rates were 0.466 and 0.431 times lower compared to patients who did not experience anosmia. In this regard, a meta-analysis by Purja et al. showed that patients with severe COVID-19 and hospitalized patients were 0.527 and 0.283 times less likely to experience anosmia compared to non-hospitalized patients, respectively (28).
The results of the logistic regression analysis showed that diabetic patients who lived in the village with higher PCO2 and BUN levels were more likely to develop the risk of the severe form of COVID-19 than others. In the study by Hu et al. (13), COVID-19-related deaths increased by 8.4 times with a decrease in CO2 levels, but it was not associated with disease severity. However, lower CO2 levels in elderly patients or those with the cardiovascular disease showed a worse prognosis. A study by Buchner et al. found that patients with CO2 retention had a worse prognosis for pneumonia (29). On the other hand, based on the logistic regression analysis results, elderly patients with pulmonary diseases with higher BUN levels were more likely to develop a risk of death than others. In the study by Wendel Garcia et al., Cr, d-dimer, lactate, K, P/F ratio, alveolar-arterial gradient, and ischemic heart disease were significantly associated with COVID-19 mortality (30). In agreement with our results, Izcovich et al. reported that some factors like age, BUN, and high neutrophil count were influential in mortality due to COVID-19 (31). In a study by Sepandi, it was reported that old age and the male gender were associated with higher mortality (32).
Elderly patients infected with COVID-19 with a history of underlying conditions suffer from a severe form of the disease with symptoms of respiratory distress, which can lead to death (33-35). In this regard, the study by Gorse et al. showed that the human Coronavirus is exacerbated by diseases like heart failure, asthma, chronic obstructive pulmonary disease (COPD), and underlying conditions, increasing the need for acute care and hospitalization in these patients (36).
In summary, it is sensible to consider the underlying conditions, including diabetes, hypertension, cardiovascular disorders, and pulmonary diseases, as well as some paraclinical and clinical parameters like FBS, BUN, Cr, Neut, SpO2, and lack of anosmia as valuable prognostic predictors of COVID-19 severity and mortality. Of note, the identification of patients who may develop severe illness and require mechanical ventilation or intensive care is essential for decreasing COVID-19 mortality rates. This will ultimately improve the efficient use of healthcare resources (37).
5.1. Conclusions
Collectively, it seems that in addition to the BUN, diabetes and pulmonary diseases play a more significant role in predicting the severity and mortality due to COVID-19, respectively.
It is suggested that other studies with a larger sample size be conducted in this field in different regions of the world.