1. Background
Bone healing is a complex biological process that can be clinically challenging (1-3). Bone defects in the jaws may originate from congenital or developmental malformation, tumor resection, cyst enucleations, trauma, infections, and tooth removal. Tooth extraction is one of the most common procedures performed in a dental office. Healing is uneventful in most cases, but some extraction wounds require longer to heal and may be painful or lead to bone defects.
Previous studies have introduced several methods to accelerate bone repair, including treating the tooth sockets with various bioactive materials (4) and using specific growth factors such as bone morphogenetic proteins (BMPs) (5), vitamin D (6), calcium phosphate (7), hormones, plant extracts (8), low-intensity ultrasound mechanical stimulation (9, 10), electromagnetic fields (11) and LLLT (12, 13). Low-level laser therapy is also known as ''photo-bio-modulation therapy" and has extensive applications in many clinical and experimental studies. Several studies have concluded that LLLT can be beneficial for several specific applications in dental practice (14). Low-level laser therapy accelerates repair with or without the administration of various osteoinductive and osteoconductive biomaterials (15). The laser affects tissues in numerous ways, such as hemostasis, microbial decontamination, tissue ablation, and vaporization, and influences biological processes, including bio-stimulation (photo-bio-modulation), which have many therapeutic effects (16). One of the promising uses of LLLT is the acceleration of bone metabolism (17). The laser applies a directional non-ionizing electromagnetic radiation, which is monochromatic and coherent (18), and its wound healing stimulation effects have been demonstrated with various power densities. Laser energy can enhance osteoblastic processes, epithelial cells and fibroblast proliferation, collagen synthesis by fibroblasts, lymphatic system activation, angiogenesis, and bone formation (19). Low-level laser therapy has also been demonstrated to boost the osteogenic properties of some biomaterials.
Bone healing takes a long time, and spontaneous bone repair can be complicated if the bone defects are more extensive than a specific size. Therefore, researchers have extensively studied the osteoinductive and osteoconductive effects of different biological and chemical agents to accelerate this process. One such biological agent is platelet-rich plasma (PRP) (20-22). There is a growing interest in applying PRP for bone repair as it reduces injured tissue's recovery time. Similarly, PRP promotes bone healing through its many bone-forming ingredients, such as specific proteins, cytokines, chemokines, and several growth factors of the plasma and platelets. Platelets have various functions, such as wound repair, re-epithelialization, and homeostasis. They also release several growth factors, stimulate angiogenesis, promote fibroblast proliferation, and accelerate collagen synthesis (23).
Platelet-rich plasma contains fibrin and various growth factors that can promote bone growth, such as vascular endothelial growth factor (VEGF), transforming growth factor-beta (TGF- β), epithelial growth factor (EGF), platelet-derived growth factor (PDGF), and fibroblast growth factor (FGF) (24). They can all be gelified as a scaffold for filling injured tissues (25). Moreover, some PRP growth factors (such as PDGF) can mobilize adjacent tissues’ mesenchymal cells to proliferate, migrate, and differentiate into osteoblasts. Previous studies have demonstrated that PRP can facilitate the migration of osteoblasts and periodontal ligament cells (26). Moreover, the synergistic effect of PRP and other biomaterials and therapeutic interventions has been confirmed in several studies (27, 28).
The combination of low-level laser and PRP has been used for stimulating regeneration in orthopedics (29-31), dermatology (using fractional CO2 laser (32, 33) and low-level laser (34)), muscle repair (35), dental implant (36), healing of periodontal defects (37), periodontal regeneration in fenestration defects (38), bone regeneration (39), and Medication-Related Osteo-Necrosis of the Jaw (MRONJ) management (40). Their combination can presumably enhance different cell regeneration processes.
2. Objectives
Considering the above gaps in the literature, the present study aimed to illuminate any potential synergic impact of biogenic materials, such as platelet-rich plasma and LLLT, on bone calcium content and tooth extraction site mechanical properties in maxillary bone defects in a rat model.
3. Methods
3.1. Ethical Statement
The University ethical committee approved the study protocol, and all procedures complied with ethical principles of animal handling and treatment (IR.SUMS.DENTAL.REC.1400.020). The ARRIVE guidelines were followed in reporting the present study.
3.2. Study Design, Sample Size, and Experimental Procedures
The current work is an experimental study. In the present study, four groups were compared: the control group, laser group, PRP group, and laser-PRP combination group. The sample size was determined according to a previous study (41) and was modified based on our limitations and constraints (ethics and time). Considering an α = 0.05 and a power of 80%, five rats were required for each group (n = 5).
The rats were procured from the Animal Laboratory of the Shiraz University of Medical Sciences. All rats were kept in large, well-controlled environments with a temperature of 22 ± 2°C and 12 h of light/dark cycles, adequate ventilation, and unlimited access to food and water.
Twenty healthy Sprague–Dawley male rats, approximately 80 - 90 days old and 180 - 200 grams, were procured from the Animal Laboratory of the University of Medical Sciences. Anesthetic methods and medications were similar in all groups.
3.3. Inclusion and Exclusion Criteria
Healthy rats were included in the study, and animals with abnormal health conditions were excluded.
3.4. Randomization and Blinding
Laboratory personnel labeled and allocated rats randomly; therefore, authors were blinded to sample groups during and at the end of the experiment.
3.5. Outcome Measures
Our primary outcome measures were the calcium content and mechanical properties of the extraction socket of rats’ first left maxillary molar.
3.6. Experimental Procedures
All rats were anesthetized by intramuscular administration of a 4:1 solution of ketamine (Vetased, Romania)/xylazine (Sedaxyl, Belgium) at a dose of 0.15ml per 100g of body weight. After the onset of anesthesia, the maxillary left first molar teeth of all animals were extracted. All animals were divided randomly into one control group (n = 5) and three experimental (case) groups: laser- (n = 5), PRP- and laser-PRP-treated (n = 5) group. In the PRP- and laser-PRP-treated groups, approximately 100 µL of PRP (after preparation) was transferred into the socket immediately after extraction, and then, 100 λ CaCl2 was added to the jellified PRP. A figure of eight sutures using vicryl 4-0 was placed over the socket to prevent the gelatinized PRP from extrusion.
The tooth sockets of both the laser group and the laser-PRP group were then exposed to gallium aluminum arsenide diode laser every 72 h after extraction for the next 12 days (4 times overall).
All rats were euthanized by CO2 70% 30 days after the extraction (42). The maxilla was harvested, and the overlying soft tissue was removed using a scalpel. Tooth sockets with a rim of peripheral bone were separated and immersed in 10% buffered neutral formalin for 48 hrs. Using a digital caliper (Digit-Cal SM, TESA S.A., Switzerland), the length and width of all bone blocks were measured as 4 ± 0.1 mm and 3 mm ± 0.1 mm, respectively. The thickness of the blocks was measured using the same caliper. All blocks were stored at -20ºC.
3.7. Preparation of PRP
Similar to our previous study (43), three mL of the rat blood was transferred into a tube containing citric acid anti-coagulant and centrifuged at 5000 rpm for 15 minutes. A micropipette was used to remove the plasma and buffy coat, which were stored in sterile tubes. Plasma was centrifuged (IntraSpin® system, BioHorizons, Birmingham, Alabama, USA) for the second time at 2000 rpm for 10 min, and the bottom one-third was considered PRP. To gelatinize PRP and activate platelets, it was fortified with 100 λ of 2.5% CaCl2 immediately after transferring into the tooth sockets and socket sutured.
3.8. Laser Device
The laser device was gallium-aluminum-arsenide (GaAlAs) (OSRAM LD 808, Germany), and its parameters were set as follows: 808 nm wavelength, 0.9 W output, 1459 J/cm2 dose, and continuous irradiation mode for five minutes (44, 45). The laser device had a beam area of 1 cm2 and a continuous wavelength of 808nm and was applied for five minutes in the vertical direction in close contact with the sockets.
3.9. Mechanical Test Assessment
All samples were simulated in terms of mass and volume. A scale with an accuracy of 0.1 mg was used to measure the mass. Archimedes' principle of solids' volume was used to evaluate the samples' volume.
The removed bone samples were longitudinally fixed for mechanical testing and exposed to axial compression mechanical tests using a Zwick/Roell machine (Germany) at a speed of 0.5mm/min.
3.10. Calcium Content Assessment
After the mechanical test, we collected all bone block pieces (generated by breaking blocks caused by the mechanical test) and prepared them for calcium content measurement. This preparation procedure started by heating in a furnace to leave the fragments of bone blocks at 520ºC for 20 h in the oven (Gallenkamp Muffle Furnace, England). After that, the ashed bones were powdered, and 0.03 grams of bone powder was dissolved in 250 µL HCl and diluted with 31 mL distilled water. The calcium contents were measured with atomic absorption (Thermo scientific iCE 3500, USA) at a wavelength of 422.7 nm.
3.11. Statistical Methods
The mechanical test data were statistically analyzed by the Mann-Whitney test. Bone calcium contents were compared by the one-way ANOVA test. Intergroup statistical differences were conducted by Fisher’s least significant difference (LSD) as post hoc tests. A P-value less than 0.05 was considered significant for calcium content and mechanical strength. All analyses were performed by SPSS 16.0 for Windows. The graph is depicted in graph pad 5.
4. Results
All rats tolerated the extraction procedure without any noticeable inflammation and were able to eat a regular diet after the procedure.
4.1. Mechanical Test
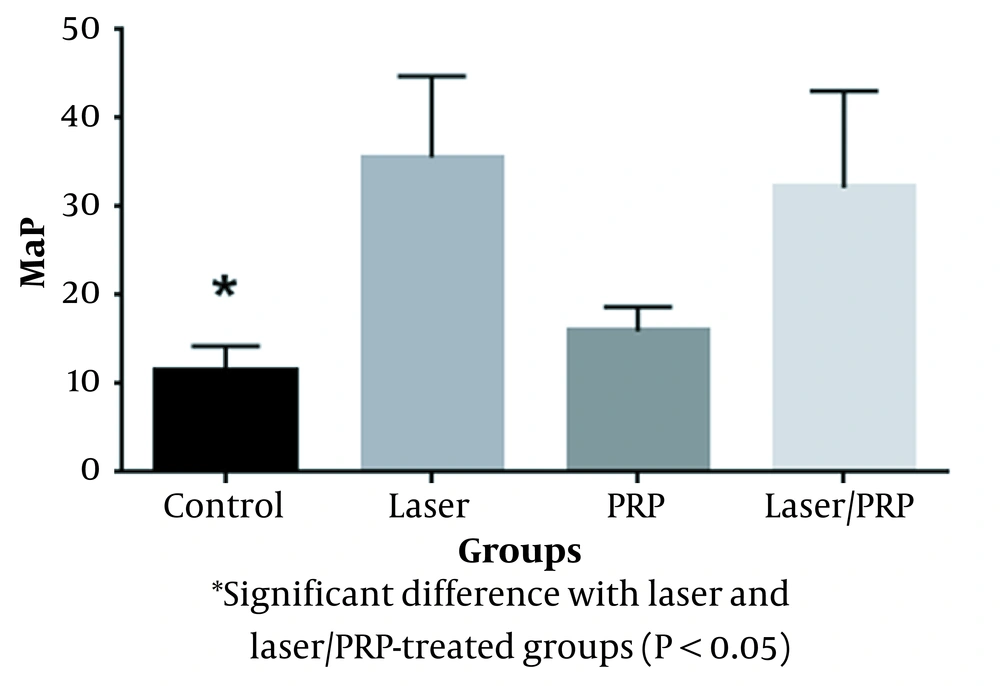
The thickness of the newly-formed bone in laser and laser/PRP groups (Range = 1.2 - 1.4 mm) was higher than the control group (Range = 0.7 - 0.8 mm).
The compressive strength in the laser group was significantly higher than in the control group (P = 0.0001, Figure 1). Also, a significant difference was observed between the laser/PRP and control groups (P = 0.00044). Moreover, the results showed that the mechanical strengths of PRP-treated and control groups were statistically similar (Figure 1).
4.2. Calcium Content Finding
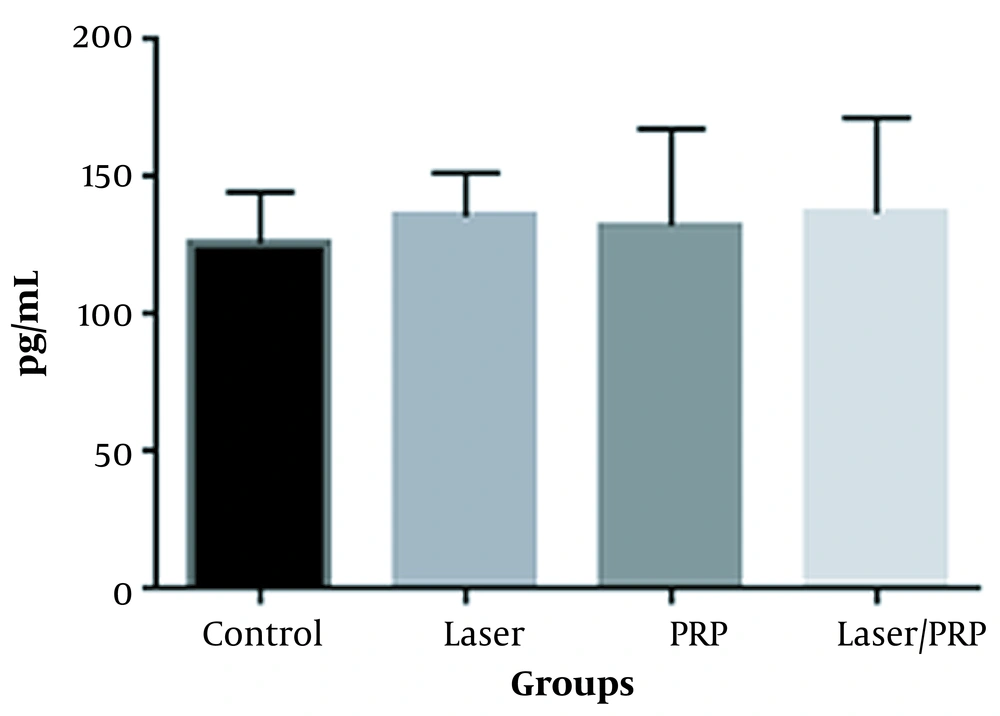
Figure 2 displays the calcium content quantity of the reparative bone in tooth sockets of different groups. The data demonstrated that neither laser nor PRP could significantly impact Ca contents. The combined laser and PRP administration also had no significant influence on Ca content (Figure 2).
5. Discussion
In the postoperative phase, bone regeneration is visible, gradually filling the defect. The bone healing process usually requires four to six months, and a longer time is needed for bone remodeling (46).
Recent studies comparing the bone-healing processes in rats and humans have observed numerous similarities, but this process is generally faster in rats (47). This was the rationale for choosing rats as the experimental model for the present study (principles of the 3Rs: replacement, reduction, and refinement) (42). A previous study demonstrated differences between human and small rodents’ bones which have the basic bone structures without Haversian canals (48).
Degranulation of the PRP promotes the discharge of several growth factors and substances such as VEGF, TGF beta-1, PDGF, FGF, connective tissue growth factor, transforming growth factors such as insulin or stimulatory (IGF-1), epidermal growth factor, platelet thromboplastin, calcium, serotonin, and fibrinogen hydrolytic enzymes (49, 50). Previous studies demonstrated that PRP could promote bone regeneration by releasing growth factors such as TGF-β1 and PDGF (22) after platelet degranulation (2).
Low-level laser therapy procedure has several limitations, such as the lack of an established optimization and a standard protocol for exposure time, power intensity, and wavelength. Moreover, different studies have used other experimental models with various exposure durations, which complicates comparing the outcomes. Low-level laser therapy effect is demonstrated to be dose-dependent, and a single exposure has no significant effect on bone repair (1, 51). In the current study, although we have applied four consecutive doses of LLLT, we observed that LLLT did not increase calcium contents.
Previous in vivo and in vitro studies demonstrated that LLLT could induce osteoblast proliferation and differentiation. Low-level laser-like GaAlAs at 830 nm wavelength stimulates osteoblastic cell growth and increases alkaline phosphatase (ALP) and osteocalcin in osteogenic cell line culture (52). ALP activity and osteoclastic expression are associated with the mineralization of newly-formed bones. The viability of osteocytes around dental implants is demonstrated to be higher after low-level laser therapy (53). Even though literature indicates a higher number of osteoblasts, higher ALP activity, and osteocalcin expression level associated with LLLT administration, our data did not show any related increase in calcium content. Therefore, the observed increase in mechanical strength seems unrelated to calcium content, increased osteoblast mineralization capacity, or ALP activity. A previous study found that LLLT can accelerate bone healing in irradiated tooth sockets of albino rats (54). It has also been demonstrated to stimulate bone formation in large calvaria defects of ovariectomized rats (3). In a recent immunohistochemical study, LLLT administration led to an increase in bone formation, collagen synthesis (55), bone metabolism, tooth movement (56), angiogenesis, and osteoid formation (57). Therefore, the observed increase in mechanical strength may be attributed to an increase in collagen content. In contrast to our findings, Nicola et al. found that applying LLLT increased mineral apposition rate and bone volume in the femur (58). The femur is a load-bearing bone, while alveolar sockets are exposed to low mechanical pressure after tooth extraction. Therefore, the different results between our findings and those of Nicola et al. can be attributed to physiological differences in mechanical stimulation.
The study of photo-bio-modulation therapy is expected to define a standardized protocol for specific wavelengths and radiation doses. The wavelength of 808 nm (used in the present study) penetrates the tissue surface (mucosa) into the underlying bone (maxilla) and may be more effective for bone-generation purposes (59). Earlier studies have demonstrated that several regenerative strategies, biomaterials, and additional therapies (such as LLLT and PRP) can accelerate bone repair and growth (1). Although we did not observe any calcium content alternation, our study’s findings indicated that LLLT administration could increase the mechanical strength of a repaired bone.
In the present study, we showed that LLLT enhances bone biomechanical properties. In addition, compared to the control group, we observed no improvement in bone biomechanical properties in the animals treated with PRP. Technically, one would expect that the combination of PRP and LLLT could reinforce bone regeneration, which would be notably superior to the application of each individual treatment (3, 22). However, we observed no significant improvement in bone biomechanical properties using a combination of LLLT/PRP treatment compared to the laser alone. In the present study, the rationale behind the increased compressive strength of newly-formed bone might be attributed to the local effects of laser stimulation of the differentiation of mesenchymal stem cells and the proliferation of fibroblasts and osteoblasts (59).
To prevent PRP removal during chewing, the edges of the tooth sockets were sutured. Suturing has been used to avoid bone exposure and the consequent necrosis that may impair bone healing (60). Our data revealed that neither PRP nor suturing (used together) demonstrated any beneficial effect on the calcium content and mechanical strength. As we did not find any PRP and LLLT synergistic effect, and noted that the PRP preparation method could influence its effects (27), the use of PRP prepared with different procedures is recommended.
5.1. Conclusions
Mechanical and biochemical analyses of the bone blocks demonstrated that PRP treatment does not improve bone biomechanical properties. However, LLLT may influence the reinforcement of bone biomechanical properties. Calcium content analysis demonstrated that PRP and LLLT, alone or in combination, were not effective.
5.2. Limitations
As PRP preparation is vital in its effects on bone repair, using just one PRP preparation method can be one of the study’s limitations. Although this method has proved effective in a previous study (43), a standard preparation protocol is lacking. Another limitation that has not been evaluated in the present study could be the evaluation of bone-specific markers, such as those involved in mineralization, which could indicate the degree of mineralization more precisely. We used the axial compression test in the present study, which was also used in previous studies to examine the biomechanical properties of bone (61). The present study examined repaired bone’s calcium content and mechanical properties, and we did not acquire histological specimens.