1. Background
There are growing numbers of elderly worldwide (1). Their life expectancy has remarkedly increased over the past century, mainly due to public health improvements (2). By 2050, the world’s population aged 60 years and above is expected to reach two billion, up from 900 million in 2015 (3). The latest general survey in Iran revealed that 7.3% of the 75 million population of Iran are aged above 60 years (4). Similarly, the population of Iran is estimated to increase by 90 million, with an increasing rate of about 30% by 2050 (5). Besides life expectancy, maintaining the quality of life (QOL) is also essential, as highlighted by the World Health Organization (WHO) and many other institutions (6). Accordingly, it is necessary to implement strategies for healthy and active aging by promoting QOL in this group of individuals (7). Quality of life is a broad-ranging concept affected in a complicated manner by individuals’ physical health, psychological state, personal beliefs, social relationships, and relationships with the salient features of the environment (4). Quality of life among the elderly tends to decline as age increases, partially due to their poorer health than the young. Aging is associated with impaired mobility, lack of social activities, depression, and low performance in daily life activities (8). In a meta-analysis of seven studies using the WHO-QOL-BRIEF questionnaire for 1366 elderly participants from Iran, the pooled mean score of total QOL was 60.1 ± 4.6. The pooled mean score of QOL was 55.13 for physical health, 51.80 for environmental, 56.68 for psychological, and 57.82 for social relationships. This study showed that men had a better QOL than women, especially in terms of physical and psychological health (9). Another review study indicated that 7% of the Iranian elderlies had high QOL, while 50 and 42% of these individuals had moderate and low levels of QOL, respectively.
2. Objectives
This study also revealed that elderlies living in nursing homes had a lower QOL compared to those living at homes and that the elderlies in the rural areas had higher levels of QOL than those in the urban areas (10). Considering the above evidence, this study aimed to investigate QOL and its relevant factors in the elderly to address modifiable variables to promote it.
3. Methods
3.1. Study Design and Sampling
This cross-sectional study was performed in 2019 - 2020 in Shiraz, the capital city of Fars Province, located in the southwest of Iran. Considering the mean level of QOL (78%) in the elders (11) at 95% confidence level and 5% error and using Cochran’s formula, the sample size was calculated to be 264. However, the final sample size was determined to be 381 considering the effect size of 1.2 (due to multistage sampling) and the dropout rate of 20%. In Shiraz, with a population of about 2 million persons, there are 172000 elderlies who are divided into three main health networks. Each health network consisted of several health centers, and due to the implementation of the family physician program, about 95% of these individuals are under the coverage of such networks. First, the sample size in each network was determined proportionally regarding their elderly population. Then the three health centers were selected using the random sampling method in each health network. The participants were selected from the existing family records regarding the proportion of registered elders in each health center using the systematic random sampling method. One elder was selected from each family, and the selected elders (or their family members) were called and informed about the study. The elders who accepted the study conditions were invited to the Motahari Clinic (Shiraz, Iran) at their convenience on one of the set dates. Those who had Alzheimer’s disease and mental diseases were excluded from this study which was indicated by their roommate in the first phone call.
3.2. Data Collection and Instruments
A face-to-face and in-person interview with each elder was performed by a same-gender interviewer in the clinic. A one-page checklist addressing the participants’ demographic, socioeconomic, and medical backgrounds was filled out for each participant by asking questions of them or their companions. The elders' Body Mass Index (BMI) was calculated according to Bassey's equation (12), and their daily intake was measured in kilocalories by analyzing their nutritional status and using a 72-hour recall checklist. Leiden-Padua (LIEPAD) questionnaire was used to assess the elders’ QOL. This questionnaire was first used in Italy (Padua and Brescia) and the Netherlands (Leiden). The questionnaire consists of two sections. The first and main section of this questionnaire addresses the core component of QOL (CCQOL), and the second section deals with the moderators of QOL (MQOL). The core component of QOL consists of 31 items, divided into seven dimensions: Physical function (5 items), self-care (6 items), depression and anxiety (4 items), cognitive function (5 items), social performance (3 items), sexual function (2 items), and life satisfaction (6 items). Each question of CCQOL is scored on a four-point Likert scale ranging from zero to three, with a maximum score of 93. The MQOL part of the LEIPAD questionnaire consists of 18 items divided into five dimensions: Perceived personality (6 items), anger (4 items), social desirability (3 items), trust in God (2 items), and self-esteem (3 items). Questions are scored on a four-point Likert scale (0 - 3) and a two-point scale (0 - 1), with a maximum score of 34. This study used the Persian version of the LEIPAD questionnaire to assess the participants’ QOL (13). Experts' opinions approved the validity of the questionnaire, and its reliability (Cronbach's alpha = 94%) was approved according to other studies (14, 15). The reliability of each of the seven components of CCQOL was also assessed in this study using Cronbach's alpha formula, and the following results were obtained: Physical function (87%), cognitive function (85%), social performance (84%), self-care (83%), sexual function (83%), life satisfaction (82%), and depression and anxiety (81%). For CCQOL, the overall Cronbach’s alpha coefficient was 0.83. We also measured the reliability of each of the five items of MQOL and reached the results below: Social desirability (89%), perceived personality (85%), self-esteem (84%), anger (81%), and trust in God (79%). The overall Cronbach’s alpha coefficient was 0.84 for MQOL. The total score of QOL was calculated by summing up the scores of CCQOL and MQOL. On this scale, scores < 50, 50 - 98, and > 98 were classified as low, moderate, and high levels of QOL, respectively.
3.3. Statistical Analysis
Data was imported to the IBM SPSS statistics software package version 25, and the accuracy of data entry was checked by selecting the data from the software randomly and matching them with the relevant questionnaires. Spearman’s tests were run to evaluate the correlations between the total score of QOL and each component of CCQOL and MQOL, between the total score of CCQOL and its components, and between the total score of MQOL and its components. The homogeneity and equality of variances were checked by Levene’s test. The univariate analysis was performed using an independent t-test to determine the factors associated with QOL, CCQOL, and MQOL. For the multivariate analysis, factors with P < 0.2 in the univariate analysis were fitted into the linear regression model (forward type). Standardized B coefficient is calculated by subtracting the mean predicted value from the predicted value, and the difference is divided by the standard deviation of the predicted values. Standardized predicted values have a mean of 0 and a standard deviation of 1. In this study, P < 0.05 was set as the significance level.
3.4. Ethics Statements
This study was conducted in accordance with the guidelines of the declaration of Helsinki. Voluntary participation in this study, the anonymous questionnaire, the possibility of access to executives of this study via two exclusive phone lines, and confidentiality in all aspects of the research were some ethical considerations. Further, the research protocol was approved by the Ethics Committee of the SUMS (Code: IR.SUMS.REC.1395.S254).
4. Results
4.1. Participants and Their Characteristics
Twenty-two out of 408 elders were excluded due to Alzheimer’s disease, and 386 (94.6%) were included in the study. The participants’ mean age was 68.12 ± 6.24 years. The participants consisted of 248 (64.2%) persons aged 60 - 69 years, 200 (51.8%) females, 272 (70.5%) married, and 350 (90.6%) individuals with 12 years of education. The mean number of individuals living with elders was 3 ± 1.5 (median = 3). The socioeconomic, demographic, anthropometric, and medical characteristics of the interviewees are presented in Table 1.
| Characteristics | No. (%) |
|---|---|
| Age (y) | |
| 60 - 69 | 248 (64.2) |
| 70 - 79 | 114 (29.5) |
| ≥ 80 | 24 (6.2) |
| Gender | |
| Male | 186 (48.1) |
| Female | 200 (51.8) |
| Education (y) | |
| ≤ 12 | 350 (90.6) |
| > 12 | 36 (9.3) |
| Education of spouse (y) | |
| ≤ 12 | 356 (92.2) |
| > 12 | 27 (6.9) |
| Marital status | |
| Married | 272 (70.4) |
| Single life | 114 (29.5) |
| Place of birth | |
| Village | 33 (8.5) |
| City | 353 (91.4) |
| Relativity with spouse | |
| 1st degree | 90 (23.3) |
| Non 1st degree | 294 (76.1) |
| Age at first marriage | |
| < 18 | 161 (41.7) |
| 18 - 24 | 113 (29.2) |
| ≥ 25 | 110 (28.4) |
| Income to cost ratio | |
| ≤ 1 | 206 (53.3) |
| > 1 | 84 (21.7) |
| Being the source of family income | |
| Yes | 222 (57.5) |
| No | 159 (41.1) |
| Supplementary insurance | |
| Yes | 281 (72.7) |
| No | 105 (27.2) |
| House ownership status | |
| Landlord | 346 (89.6) |
| Tenant | 40 (10.3) |
| Being the main decision maker about family finances | |
| Yes | 254 (65.8) |
| No | 132 (34.1) |
| Occupation | |
| Yes | 43 (11.1) |
| No | 343 (88.8) |
| Tobacco smoking | |
| Yes | 44 (11.3) |
| No | 342 (88.6) |
| Daily calories intake (kcal) | |
| < 1600 | 266 (68.9) |
| ≥ 1600 | 117 (30.3) |
| Cardiac diseases | |
| Yes | 66 (17) |
| No | 320 (82.9) |
| Hypertension | |
| Yes | 216 (56) |
| No | 170 (44) |
| BMI kg/m2 (Bassey method) | |
| Mean ± SD (median) | 25.8 ± 5.0 (25.6) |
| Migraine | |
| Yes | 77 (19.9) |
| No | 309 (80.1) |
| Psychological disease | |
| Yes | 84 (21.7) |
| No | 302 (78.2) |
| Chronic pain | |
| Yes | 259 (67) |
| No | 127 (32.9) |
| Renal diseases | |
| Yes | 78 (20.2) |
| No | 308 (79.7) |
| Osteoporosis | |
| Yes | 175 (45.3) |
| No | 211 (54.6) |
| Hematological diseases | |
| Yes | 65 (16.8) |
| No | 321 (83.1) |
| Auditory impairment | |
| Yes | 378 (97.9) |
| No | 8 (2) |
| Diabetes mellitus | |
| Yes | 119 (30.8) |
| No | 267 (69.1) |
| Hyperlipidemia | |
| Yes | 184 (47.6) |
| No | 202 (52.3) |
| Sexual problems | |
| Yes | 58 (15) |
| No | 328 (84.9) |
| Inability to walk | |
| Yes | 46 (11.9) |
| No | 340 (88) |
| Taking medical supplement | |
| Yes | 346 (89.6) |
| No | 40 (10.3) |
a Missing value is not shown in this table.
4.2. Total Scores of Qualities of Life, Core Component of Quality of Life, and Moderators of Quality of Life
The mean score of QOL was 83.67 ± 13.75 (out of 127), representing a moderate level of QOL among the elders. Accordingly, 12 (0.5%) participants had low QOL, while 281 (72.8%) and 84 (21.9%) persons reported moderate and high levels of QOL, respectively. For nine elderlies, QOL could not be categorized since they had not answered the questionnaire completely. The total score of CCQOL was 70.68 ± 9.42 (out of 93), with an interquartile range (IQR) from 66 to 77. The total score of MQOL was 20.94 ± 2.30 (out of 34), with an IQR from 19 to 22.
4.3. Correlates of Quality of Life
The univariate analyses showed that female gender, single life, level of education (up to 12 years), unemployment (elderlies and their spouses), and not being the source of family income had a negative statistical relationship with the total score of QOL (Table 2). Moreover, the history of chronic diseases (e.g., vascular, skeletal, and neurological diseases, sleep-related disorders, and hyperlipidemia), chronic pain, facing violence, and not taking medical supplements had an inverse correlation with the total score of QOL (P < 0.2; Table 2). Other socioeconomic, demographic, anthropometric, and medical characteristics of the elders were not correlated with the total score of QOL (P > 0.2). The multivariate analyses showed that sleep disorders (B = -0.15), osteoporosis (B = -0.14), female gender (B = -0.13), and not being the source of family income (B = -0.13) had a significant and inverse relationship with the total score of QOL (P < 0.05) (Table 3).
| Characteristics | CCQOL | MQOL | QOL |
|---|---|---|---|
| Gender | |||
| Male | 72.5 ± 8.1 | 14.4 ± 2.1 | 86.0 ± 11.7 |
| Female | 68.9 ± 10.1 | 13.8 ± 2.6 | 81.4 ± 15.0 |
| P-value | < 0.01 | 0.031 | < 0.001 |
| Marital status | |||
| Single/single life | 69.5 ± 10.4 | 13.9 ± 2.6 | 81.6 ± 12.4 |
| Married | 71.1 ± 8.9 | 14.2 ± 2.3 | 84.5 ± 12.4 |
| P-value | 0.120 | 0. 372 | 0.060 |
| Education (y) | |||
| ≤ 12 | 83.4 ± 14.0 | 14.1 ± 2.5 | 80.9 ± 15.7 |
| > 12 | 86.2 ± 9.9 | 14.2 ± 1.8 | 85.1 ± 10.5 |
| P-value | 0.244 | 0.698 | 0.021 |
| Education of spouse (y) | |||
| ≤ 12 | 83.8 ± 13.1 | 14.1 ± 2.4 | 80.6 ± 15.3 |
| > 12 | 86.5 ± 8.9 | 14.1 ± 2.3 | 84.5 ± 12.3 |
| P-value | 0.286 | 0.902 | 0.024 |
| Employment | |||
| Yes | 72.8 ± 5.7 | 14.1 ± 2.4 | 86.9 ± 6.8 |
| No | 70.4 ± 9.7 | 14.1 ± 2.3 | 83.2 ± 14.3 |
| P-value | 0.022 | 0.951 | 0.011 |
| Spouse having job | |||
| Yes | 84.6 ± 11.6 | 14.1 ± 2.4 | 70.9 ± 9.0 |
| No | 80.3 ± 18.9 | 13.9 ± 2.5 | 69.3 ± 11.1 |
| P-value | 0.131 | 0.501 | 0.269 |
| Being the source of family income | |||
| Yes | 72.2 ± 8.5 | 14.3 ± 2.2 | 85.9 ± 11.6 |
| No | 68.6 ± 10.0 | 13.8 ± 2.6 | 80.7 ± 15.7 |
| P-value | < 0.001 | 0.023 | < 0.001 |
| Supplementary insurance | |||
| Yes | 84.7 ± 12.2 | 14.3 ± 2.2 | 70.9 ± 9.6 |
| No | 80.7 ± 16.8 | 13.5 ± 2.8 | 69.8 ± 8.7 |
| P-value | 0.206 | 0.003 | 0.312 |
| Inability to walk | |||
| Yes | 65.5 ± 12.8 | 13.1 ± 3.1 | 65.5 ± 12.8 |
| No | 71.3 ± 8.6 | 14.2 ± 2.3 | 71.3 ± 8.6 |
| P-value | 0.004 | 0.020 | 0.004 |
| Sleep disorder | |||
| Yes | 68.2 ± 9.4 | 13.8 ± 2.5 | 81.1 ± 13.3 |
| No | 73.6 ± 8.5 | 14.4 ± 2.2 | 86.8 ± 13.6 |
| P-value | < 0.001 | 0.012 | < 0.001 |
| Chronic pain | |||
| Yes | 69.3 ± 9.6 | 13.9 ± 2.5 | 82.4 ± 13.2 |
| No | 73.3 ± 8.4 | 14.5 ± 2.1 | 86.1 ± 14.4 |
| P-value | < 0.001 | 0.024 | 0.011 |
| Taking medical supplements | |||
| Yes | 74.5 ± 6.2 | 14.2 ± 1.8 | 84.7 ± 12.2 |
| No | 70.2 ± 9.6 | 14.1 ± 2.5 | 80.7 ± 16.8 |
| P-value | < 0.001 | 0.804 | 0.030 |
| Dermatological diseases | |||
| Yes | 81.0 ± 11.3 | 13.0 ± 3.0 | 67.9 ± 9.1 |
| No | 83.8 ± 13.8 | 14.2 ± 2.4 | 70.8 ± 9.4 |
| P-value | 0.336 | 0.034 | 0.147 |
| Sexual problem | |||
| Yes | 81.8 ± 10.9 | 13.0 ± 2.7 | 69.0 ± 9.5 |
| No | 84 ± 14.1 | 14.3 ± 2.3 | 70.9 ± 9.3 |
| P-value | 0.276 | 0.001 | 0.149 |
| Migraine | |||
| Yes | 67.0 ± 11.6 | 13.4 ± 2.4 | 79.6 ± 15.0 |
| No | 71.6 ± 8.5 | 14.3 ± 2.4 | 84.6 ± 13.2 |
| P-value | 0.002 | 0.008 | < 0.001 |
| Hyperlipidemia | |||
| Yes | 69.1 ± 9.9 | 14.0 ± 2.5 | 82.0 ± 14.3 |
| No | 72.1 ± 8.7 | 14.2 ± 2.3 | 85.1 ± 13.0 |
| P-value | 0.002 | 0.578 | 0.035 |
| Rheumatic diseases | |||
| Yes | 66.2 ± 11.7 | 14.0 ± 3.0 | 66.2 ± 11.7 |
| No | 70.8 ± 9.3 | 14.1 ± 2.4 | 70.8 ± 9.3 |
| P-value | 0.116 | 0.949 | 0.116 |
| Severe weight loss | |||
| Yes | 67.8 ± 10.5 | 13.7 ± 2.6 | 67.8 ± 10.5 |
| No | 70.8 ± 9.3 | 14.1 ± 2.4 | 70.8 ± 9.3 |
| P-value | 0.117 | 0.428 | 0.117 |
| Facing violence | |||
| Yes | 67.8 ± 10.5 | 13.6 ± 2.4 | 81.5 ± 11.9 |
| No | 72.7 ± 7.8 | 14.5 ± 2.3 | 85.2 ± 14.7 |
| P-value | < 0.001 | < 0.001 | < 0.001 |
| Hematological disease | |||
| Yes | 68.5 ± 8.2 | 13.6 ± 2.6 | 68.5 ± 8.2 |
| No | 71.1 ± 9.6 | 14.2 ± 2.4 | 71.1 ± 9.5 |
| P-value | 0.003 | 0.108 | 0.353 |
| Hepatic disease | |||
| Yes | 82.5 ± 13.3 | 13.3 ± 2.3 | 69.2 ± 11.9 |
| No | 83.8 ± 13.8 | 14.2 ± 2.4 | 70.8 ± 9.0 |
| P-value | 0.603 | 0.036 | 0.312 |
| Osteoporosis | |||
| Yes | 68.4 ± 10.3 | 13.9 ± 2.5 | 80.8 ± 15.4 |
| No | 72.4 ± 8.1 | 14.3 ± 2.3 | 86 ± 11.6 |
| P-value | < 0.001 | 0.103 | < 0.001 |
| Cardiac disease | |||
| Yes | 81.3 ± 13.9 | 14.1 ± 2.4 | 68.1 ± 9.6 |
| No | 84.1 ± 13.6 | 14.2 ± 2.3 | 71.2 ± 9.8 |
| P-value | 0.126 | 0.705 | 0.215 |
| Hypertension | |||
| Yes | 69.5 ± 10.0 | 14.1 ± 2.4 | 82.0 ± 14.3 |
| No | 72.1 ± 8.3 | 14.1 ± 2.4 | 84.9 ± 13.3 |
| P-value | 0.005 | 0.984 | 0.102 |
| Psychological diseases | |||
| Yes | 80.8 ± 10.9 | 13.8 ± 2.4 | 67.0 ± 9.4 |
| No | 84.4 ± 14.3 | 14.2 ± 2.4 | 71.7 ± 9.1 |
| P-value | 0.032 | 0.166 | 0.207 |
| Renal diseases | |||
| Yes | 69.2 ± 11.9 | 13.9 ± 2.0 | 68.7 ± 10.0 |
| No | 70.8 ± 9.0 | 14.1 ± 2.5 | 71.1 ± 9.1 |
| P-value | 0.042 | 0.550 | 0.214 |
Abbreviations: CCQOL, core components of quality of life; MQOL, moderators of quality of life; QOL, quality of life.
| Characteristic | Standardized ß | P | Unstandardized ß | 95% CI |
|---|---|---|---|---|
| QOL | ||||
| Sleep disorders | -0.15 | < 0.001 | -4.09 | (-5.86, -2.32) |
| Osteoporosis | -0.14 | < 0.001 | -3.25 | (-7.90, -2.59) |
| Gender (female/male) | -0.13 | < 0.001 | -3.69 | (-5.47, -1.91) |
| Not being the source of family income | -0.13 | 0.042 | -1.86 | (-3.66, -0.06) |
| CCQOL | ||||
| Sleep disorders | -0.21 | 0.028 | -4.01 | (-6.58, -1.43) |
| Facing violence | -0.21 | 0.031 | -2.16 | (-5.34, -0.25) |
| Gender (female/male) | -0.17 | 0.007 | -4.11 | (-7.10, -1.11) |
| Migraine | -0.14 | 0.006 | -5.68 | (-9.72, -1.63) |
| Psychological disease | -0.13 | 0.012 | -0.95 | (-2.48, -0.80) |
| Not being the source of family income | -0.09 | 0.018 | -2.37 | (-5.73, -0.53) |
| MQOL | ||||
| Sexual problems | -0.17 | 0.001 | -1.15 | (-1.82, -0.48) |
| Facing violence | -0.16 | 0.001 | -0.80 | (-1.82, -0.48) |
| No supplementary insurance coverage | -0.15 | 0.002 | -0.83 | (-1.36, -0.30) |
| Inability to walk | -0.14 | 0.006 | -1.02 | (-1.76, -0.29) |
| Migraine | -0.12 | 0.013 | -0.76 | (-1.37, -0.16) |
Abbreviations: P, P-value; CI, confidence interval; QOL, quality of life; CCQOL, core components of quality of life; MQOL, moderators of quality of life.
4.4. Correlates of CCQOL
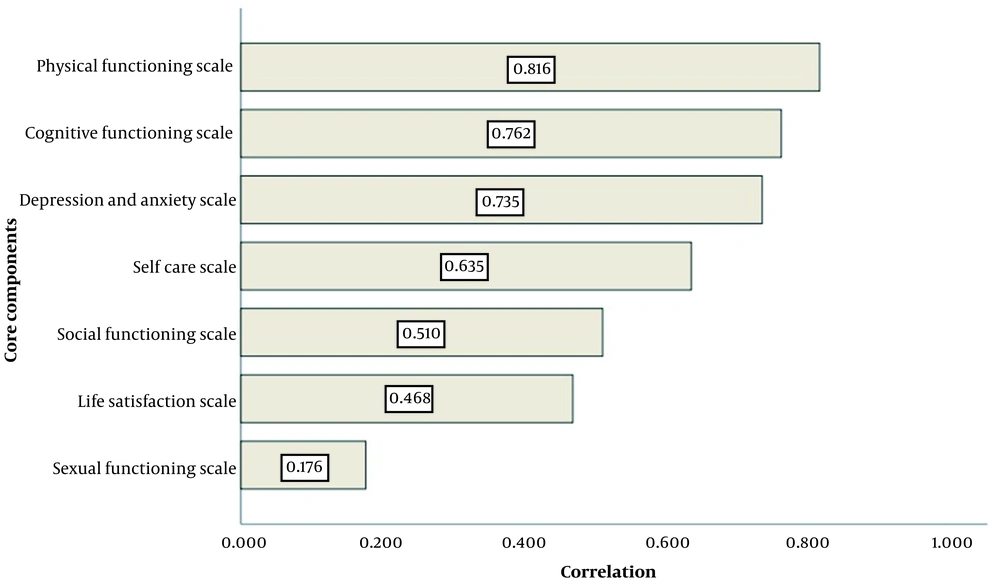
Figure 1 demonstrates the relationship between the total score of CCQOL with each of its seven components. Physical functioning and sexual functioning (0.17) had the strongest and weakest correlation (0.81) with the total score of CCQOL.
The univariate analyses showed that the female gender, single life, non-employment, and not being the source of family income had a negative relationship with the total score of CCQOL (Table 2). Further, a history of chronic diseases (e.g., cardiovascular, hematological, renal, skeletal, neurological, rheumatic, and psychological diseases, sleep disorders, and hyperlipidemia), chronic pain, facing violence, and taking medical supplements had an inverse correlation with CCQOL (P < 0.2; Table 2). Other socioeconomic, demographic, anthropometric, and medical characteristics of the elders were not correlated with the QOL score (P > 0.2). Multivariate analyses showed that sleep disorders (B = -0.21), facing violence (B = -0.21), female gender (B = -0.17), migraine (B = -0.14), psychological diseases (B = -0.13), and not being the source of family income (B = -0.09) had significant and inverse correlations with CCQOL (P < 0.05; Table 3).
4.5. CCQOL Components
4.5.1. Physical Functioning
The mean score of physical function was 11.3 ± 2.6 (out of 15). Sleep disorders (B = -0.20), chronic pain (B = -0.18), female gender (B = -0.16), osteoporosis (B = -0.12), low age of marriage (B = -0.12), and migraine (B = -0.11) had negative and significant relationships with the elders’ physical functioning.
4.6. Cognitive Functioning
The mean score of cognitive function was 12.1 ± 2.5 (out of 15). Sleep disorders (B = -0.20), migraine (B = -0.17), and facing violence (B = -0.14) had a significant relationship with a decrease in cognitive functioning.
4.7. Depression and Anxiety
The mean score of depression and anxiety was 9.4 ± 2.9 (out of 12). Migraine (B = 0.19), psychological diseases (B = 0.17), female gender (B = 0.16), sleep disorders (B = 0.14), and facing violence (B = 0.11) had significant and positive relationships with the elders’ depression and anxiety levels.
4.8. Self-care
The mean self-care score was 16.6 ± 2.3 (out of 18). Female gender (B = -0.19), older age (B = -0.19), osteoporosis (B = -0.15), facing violence (B = -0.10), and hypertension (B = -0.10) significantly decreased the elders’ self-care.
4.9. Social Functioning
The mean score of social function was 6.6 ± 2.0 (out of 9). Facing violence (B = -0.25), single life (B = -0.19), female gender (B = -0.13), and not being the source of family income (B = -0.13) had significant and inverse relationships with the elders’ social functioning.
4.10. Life Satisfaction
The mean score of life satisfaction was 10.30 ± 2.36 (out of 18). Facing violence (B = -0.18), sexual problems (B = -0.15), sleep disorder (B = -0.12), and not being the source of family income (B = -0.15) decreased the elders’ life satisfaction.
4.11. Sexual Functioning
The mean score of sexual function was 4.1 ± 1.6 (out of 6). Single life (B = -0.66), female gender (B = -0.18), sexual problems (B = -0.16), and older age (B = -0.14) had significant and inverse relationships with the elders’ sexual functioning.
4.12. MQOL Components and It Is Correlates
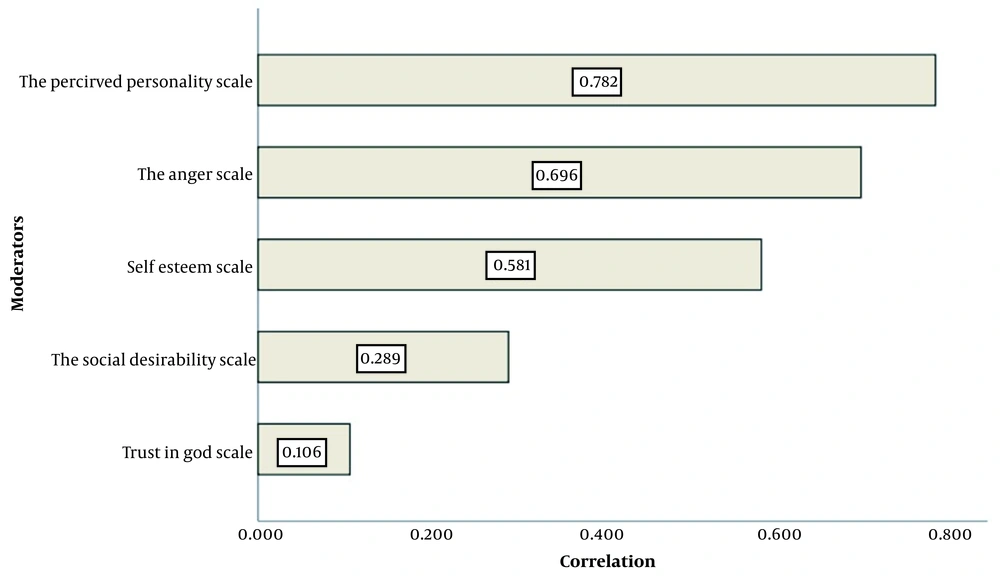
The mean scores were 4.02 ± 1.22 (out of 8) for and t 9.20 ± 1.06 (out of 12) for anger, 0.28 ± 0.55 (out of 3) for social desirability, 6.42 ± 0.79 (out of 9) for self-esteem, and 1.0 ± 0.07 (out of 2) for trust in God. Among the five items of MQOL, perceived personality (0.78) and trust in God (0.1) had the highest and the lowest correlation with the total score of MQOL, respectively (Figure 2). The univariate analysis showed inverse and significant relationships between the MQOL with female gender, no supplementary insurance coverage, not being the source of family income, and sleep disorder (Table 2). Moreover, a history of chronic diseases (e.g., liver diseases, psychological diseases, osteoporosis, skin diseases, migraine, hematological diseases, inability to walk, and sexual problems), chronic pain, and facing violence had inverse correlations with MQOL (P < 0.2; Table 2). Multivariate analyses showed inverse relationships between MQOL with sexual problems (B = -0.17), facing violence (B = -0.16), no supplementary insurance coverage (B = -0.15), inability to walk (B = -0.14), and migraine (B = -0.12) (P < 0.05; Table 3).
5. Discussion
The findings of this study revealed that at least seven out of ten elders participating in this study had a moderate level of QOL. Moreover, elderly women, elders with chronic diseases (sleep disturbance, osteoporosis), and those who were not the source of their family income had a lower level of QOL. Moreover, facing violence was inversely correlated with both CCQOL and MQOL in the elders and sexual problems. No supplementary insurance coverage decreased the elders’ MQOL. Among chronic diseases, sleep disturbance had the most inverse relationship with QOL and CCQOL, while sexual problems had the strongest relationship with MQOL. Furthermore, physical functioning and perceived personality had the strongest correlation with CCQOL and MQOL, respectively.
Population aging is one of the most challenging public health issues in today's world (16); as a result, the prevalence of chronic diseases and the need to care for elderly adults have increased (17). On the other hand, achieving a higher level of QOL in the elderly has been more concerned by policymakers compared to solely raising their life expectancy (18). However, unlike developed countries, developing countries are not prepared enough to manage the health, social, and economic consequences of aging (4).
A meta-analysis study in Iran showed that elderly men had higher levels of QOL than older women (8). This finding was in line with the findings of the present study. Another review study in Iran revealed that 50% of elderlies had a moderate level of QOL (10).; the reported value was below the value reported for the moderate level of QOL in the present study (70%).
Zeitlhofer et al. in Austria reported a significant relationship between sleep quality and the total score of QOL. They suggested that poor sleep quality could be used as a screening method in examining patients' QOL (19), which is in line with our findings. The relationship between quality of sleep and QOL was also documented in another study (20).
Similar to our findings, Lips, and van Schoor (21) and Madureira et al. (22) found a relationship between osteoporosis and lower levels of QOL in the elders. Lee and Shinkai revealed that gender was not correlated with QOL (23), which is in contrast to our findings. Lemos et al. also concluded that QOL was lower in elderly females than in elderly males (24). In contrast to some other studies (25, 26), we found no association between the low level of income and QOL; however, there was a positive association between being the source of family income and QOL. This finding has not been reported in other studies. We also showed that the place of birth (urban vs. rural) of the elders and their spouses had no relationship with their QOL. This finding is inconsistent with those from another study conducted in Spain (27). In recent years, scientists have been more concerned with different aspects of sexuality as the determinants of QOL in the elders. Flynn and Gow concluded that sexual function is a significant predictor of QOL in elderly adults (28). We also concluded that sexual function was positively correlated with MQOL in the elders. Moreover, the prevalence of depression has increased in many countries, including the Middle East, over the past two decades (29). Similar to our study, Canuto et al. indicated that the incidence of depression and anxiety disorders had a negative relationship with QOL in the elders, especially in women (30). In another study, violence toward elders was associated with their lower levels of QOL, as we reported in this study (31). Brovold et al. showed that physical function had a significant relationship with aging, implying that it is important to encourage elders to be physically active and thereby promote their QOL (32). Similarly, physical function was the strongest predictor of CCQOL among the other components. Our study had some limitations and strengths. We could not assess the QOL of those elders who were not under the coverage of health centers. However, the participants of this study consisted of only about 5% of all elders in Shiraz city. Moreover, there are limited nursing homes in this city. Another point is that to detect causal relationships between QOL and other factors, further longitudinal studies are recommended. On the other hand, as one of the strengths of this study, the present research was among scarce studies assessing MQOL and its relationship with QOL in the elders. Furthermore, we also measured the correlation between different socioeconomic, demographic, anthropometric, and medical backgrounds with QOL, CCQOL, and MQOL. Further, the correlations between the components of CCQOL and MQOL with their relevant scores were also investigated.
5.1. Conclusions
Four out of five elders in the Shiraz region had a moderate level of QOL, while the elder females and the elders with chronic diseases, especially those with sleep disorders or osteoporosis, as well as the elders who were not the source of their family income, had lower levels of QOL. Facing violence also had an association with lower scales of both CCQOL and MQOL, while sexual problems and n no supplementary insurance coverage decreased MQOL. Accordingly, QOL in the elders, especially elderly females, can be improved by offering an integrated care program in the health centers to change or modify the above conditions; however, the role of the elders’ families in this regard should not be overlooked.