1. Background
Abortion means terminating a pregnancy before the twentieth week through medical or surgical means (1). It may be utilized as a treatment when the mother's or fetus's health is at risk (therapeutic abortion) or when a woman desires pregnancy termination due to an unexpected pregnancy (elective abortion). Globally, there are around 205 million pregnancies yearly, and more than a third of them are unplanned, with one-fifth of mothers desiring an induced abortion (2, 3). Most abortions are performed to avoid unwanted pregnancies, while most therapeutic abortions aim to prevent the birth of a child with physical, metabolic, or cognitive abnormalities (4). The choice of procedure depends on the gestational age and the patient's preferences. The success rate for surgical abortions during the first trimester is approximately 98 percent, with a complication rate of about 7.8 percent (5). The success rate of medical abortions is not as high as that of surgical abortions, and approximately 4 to 10 percent of patients require curettage due to an incomplete abortion. One advantage of medical abortion is that it does not require surgery or anesthesia. However, a disadvantage is the time needed to complete the process and the likelihood of an incomplete abortion (6, 7).
Dilation and curettage used to be a primary therapy for first-trimester abortion, but it came with risks such as uterine rupture, excessive bleeding, and infection (8). Therefore, non-invasive medicinal therapies have been advocated as an acceptable treatment in recent decades. Among them, the most common approach is mifepristone with misoprostol or misoprostol alone, and hygroscopic dilators may be used in conjunction with this approach (9, 10). In addition to treating gastric ulcers, misoprostol improves cervical effusion and induces uterus contractions (11). Letrozole, a third-generation non-steroidal aromatase inhibitor, has a half-life of approximately 45 hours and is eliminated from the body through the liver. Its anti-estrogenic properties treat breast cancer (12). Recent trials have used letrozole in conjunction with misoprostol to induce abortion. By reducing estrogen production from the corpus luteum, this medication can assist in inducing abortion.
2. Objectives
This study aimed to compare the success rate of medical abortions between misoprostol/letrozole and misoprostol/placebo.
3. Methods
This double-blind, randomized clinical trial was conducted on pregnant women who were candidates for medical abortion at the teaching hospitals of Mashhad University of Medical Sciences between 2018 and 2019. The inclusion criteria were: Age over 18 years, gestational age under twenty weeks as determined by ultrasound, hemoglobin level above 10 dl/mg, lower diastolic pressure less than 95 mmHg, no history of thromboembolism, malignancy, or liver disease, no history of asthma or porphyria, and no intrauterine device (IUD) in place. The exclusion criteria included drug sensitivity and patient refusal to participate.
3.1. Study Design
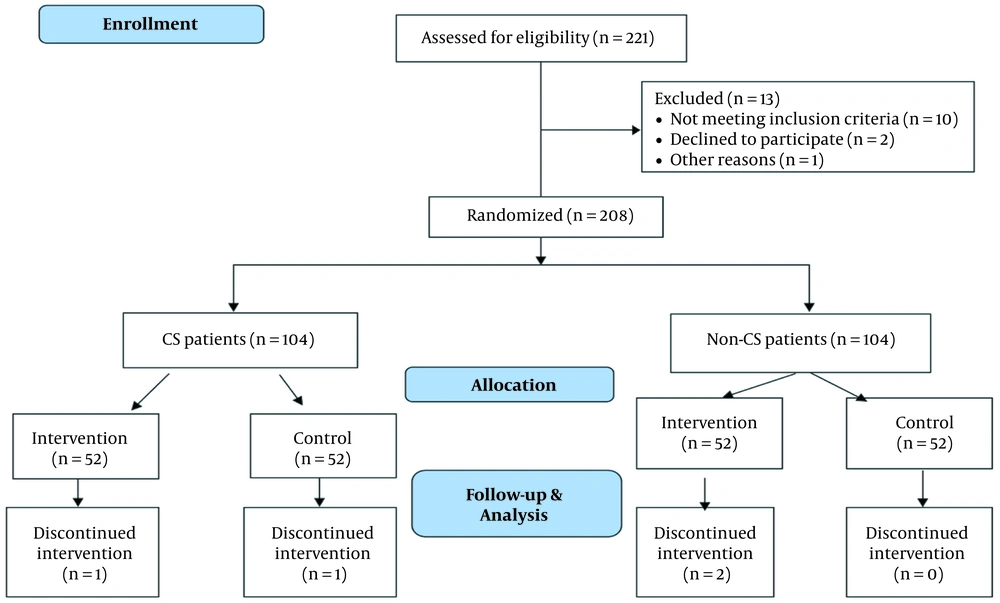
After obtaining written informed consent, eligible women were separated into two groups based on their history of cesarean section. The groups were randomly assigned to either the intervention or control group within each subgroup. A computer software-generated list of 104 numbers was used for each subgroup. Each number corresponded to a patient and was written on a prepared envelope containing either four pieces of letrozole or four pieces of placebo. The patients were instructed on taking the medication, and both the patient and the nurse who provided the envelope were unaware of the group to which the patient was assigned. In the cesarean section group, 52 patients were included in each group. The intervention group was given letrozole 2.5 mg every 6 hours (manufactured by Iran Hormone Company) two days before admission. The third dose of letrozole was taken at the time of admission. Misoprostol was administered vaginally to both groups after admission, and the dosing was adjusted based on gestational age and national norms. Adverse symptoms such as fever and chills, nausea and vomiting, headache, and rash were monitored by a midwife unaware of the group assignment. Patients who underwent abortion were evaluated with ultrasound 12 hours after the abortion to ensure complete abortion. An endometrial thickness of less than 15 mm was defined as complete abortion, while a thickness greater than 15 mm was considered incomplete and required curettage. The consort diagram is shown in Figure 1.
3.2. Outcomes
The primary outcome of this study was the rate of complete abortion in each group. The secondary outcomes were the total dose of misoprostol used for complete abortion and the time interval between the first misoprostol dose and abortion.
3.3. Statistical Analysis
The collected data were analyzed using SPSS version 16 software. Descriptive statistical methods, including central indicators, dispersion, and frequency distribution, were used to overview the data in tables and graphs. An independent t-test was used to compare quantitative variables between the two groups when the data were normally distributed, while the Mann-Whitney test was used otherwise. The chi-square test and Fisher's exact test were used to analyze qualitative factors between the two groups. The significance level for all analyses was set at 0.05. The sample size was determined based on the similar study (13), which provided the necessary variables for computing the sample size. With a significance level of α = 0.05, power of β = 0.1, P1 = 76.7%, and P2 = 42.6%, a sample size of 41 participants was calculated for each group using the PASS program. Fifty-two participants were included in each group, comprising 208 participants, to account for a hypothetical 20% dropout rate.
3.4. Ethics
The ethics committee approved the study protocol recorded on the clinical trial site. All patients completed the informed consent form and were removed from the trial if unsatisfied. Patients were also assured that all their information would be evaluated anonymously, and their identities would not be disclosed in the research. This study was approved by the Ethics Committee of Mashhad University of Medical Sciences with the code IR.MUMS.fm. REC.1395.569. The study protocol was registered in IRCT with IRCTID: IRCT2017042933680N1 (Link: https://fa.irct.ir/trial/25916).
4. Results
The study results are presented in two sections: The group with a previous history of cesarean section (CS group) and the group with no history of cesarean section (non-CS group).
As presented in Table 1, the CS group consisted of 52 patients in the intervention group and 52 in the control group. The mean age in the control and intervention groups was 31.59 ± 5.6 and 31.06 ± 4.6, respectively, and no statistically significant difference was found between the two groups (P = 0.605). Similarly, the mean gestational age, determined by sonography, in the control and intervention groups was 11.20 ± 3.3 and 10.29 ± 2.6 weeks, respectively, which did not show a statistically significant difference between the two groups (P = 0.135). Additionally, the two groups had no significant difference in systolic blood pressure (P = 0.66).
| Characteristics | Control Group (N = 51) | Intervention Group (N = 51) | P-Value |
|---|---|---|---|
| Age (y) | 31.59 ± 5.6 | 31.06 ± 4.6 | 0.605 |
| Gestational age by ultrasound (weeks) | 11.20 ± 3.3 | 10.29 ± 2.6 | 0.135 |
| Blood pressure (mmHg) | 107.06 ± 4.6 | 107.45 ± 4.4 | 0.66 |
a Values are expressed as Mean ± SD.
Furthermore, the study compared the rate of complete abortion between the two groups. The results showed that the rate of complete abortion in the intervention group was significantly higher than in the control group (P = 0.001). There was no significant difference between the misoprostol dose in the control group and the intervention group in the CS group (P = 0.111). However, there was a significant difference in the time interval between the first misoprostol dose and abortion between the two groups (P < 0.001) (Table 2).
| Control Group (N = 51) | Intervention Group (N = 51) | P-Value | |
|---|---|---|---|
| Complete abortion | 0.001 | ||
| Yes | 12 (23.5) | 28 (54.9) | |
| No | 39 (76.5) | 23 (45.1) | |
| Misoprostol dose (mg) | 1039.22 ± 583.8 | 1223.53±573.6 | 0.111 |
| The time interval between the first misoprostol dose and abortion | 10.71 ± 2.5 | 6.65 ± 2.1 | < 0.001 |
a Values are expressed as No. (%) or Mean ± SD.
In the non-CS group, the study included 52 patients in the control group and 50 in the intervention group. The mean age in the control and intervention groups was 29.50 ± 7.4 and 27.8 ± 6.16, respectively. There was no statistically significant difference between the two groups (P = 0.078). The mean gestational age by ultrasound in the control and intervention groups was 9.88 ± 3.9 and 9.46 ± 2.09 weeks, respectively, showing no statistically significant difference (P = 0.500). However, a significant difference was found between the systolic blood pressure of the two groups (P = 0.356) (Table 3).
| Characteristics | Control Group (N = 52) | Intervention Group (N = 50) | P-Value |
|---|---|---|---|
| Age (y) | 29.50 ± 7.4 | 27.8 ± 6.16 | 0.078 |
| Gestational age by ultrasound (weeks) | 9.88 ± 3.9 | 9.46 ± 2.09 | 0.500 |
| Blood pressure (mmHg) | 107 ± 4.6 | 107.80 ± 4.98 | 0.356 |
a Values are expressed as Mean ± SD.
As shown in Table 4, the rate of complete fetal excretion in the intervention group was significantly higher than that of the control group in the non-CS patients. The complete abortion rate was higher in the intervention group (P = 0.008). There was no significant difference between the misoprostol dose in the control group and the intervention group in the non-CS group (P = 0.172). However, there was a significant difference in the time interval between the first misoprostol dose and abortion between the two groups (P = 0.003).
| Control Group (N = 52) | Intervention Group (N = 50) | P-Value | |
|---|---|---|---|
| Misoprostol dose (mg) | 1026.92 ± 453.36 | 1168.00 ± 573.36 | 0.172 |
| The time interval between the first misoprostol dose and abortion | 8.12 ± 2.25 | 6.78 ± 2.25 | 0.003 |
| Complete abortion | 24 (46.2) | 36 (72.0) | 0.008 |
a Values are expressed as Mean ± SD or No. (%).
The study did not observe any side effects in any of the groups, including nausea, vomiting, fever, or rash.
5. Discussion
Compared to surgery, abortion has fewer adverse effects, such as bleeding and infection, and it puts less stress on patients (13). Inducing abortion is feasible using various medication regimens. Misoprostol is a relatively safe and affordable choice, a prostaglandin analog used to induce abortion. Letrozole, on the other hand, is a non-steroidal third-generation aromatase inhibitor with an average half-life of about 45 hours, eliminated in the urine. Possible adverse effects include edema, headache, and dizziness. Letrozole is contraindicated during pregnancy and breastfeeding (14, 15). This medication can aid in the induction of abortion by decreasing estrogen production in the corpus luteum (16).
Previous studies have reported similar findings to the present study. In 2018, Abbasalizadeh et al. examined the effect of letrozole combined with misoprostol against misoprostol alone on the incidence of miscarriage in the first trimester. Their findings indicated that complete abortion occurred in 93.7 percent of the intervention group and 68.7 percent of the control group. They concluded that letrozole medication during the first trimester, combined with misoprostol, can raise the rate of complete abortion without raising adverse effects (17). Their study findings were in line with the present study. In a pilot randomized double-blind trial by Jain et al., researchers compared abortion using mifepristone in combination with misoprostol and a misoprostol-alone regimen. They concluded that complete abortion success rates were considerably greater with mifepristone and misoprostol than with the misoprostol-alone regimen (18). In a 2011 study, Lee et al. examined the use of letrozole in combination with misoprostol or mifepristone for second-trimester abortion (16). The results indicated that both groups had a similar rate of abortion at 24 and 48 hours, which is in contrast to our findings. Yeung et al. (19) and Naghshineh et al. (20) conducted research that revealed supporting results. Additionally, Lee et al. conducted a pilot trial to explore the use of letrozole in combination with misoprostol or mifepristone for the termination of pregnancy for up to 63 days. According to their findings, letrozole with misoprostol may be advantageous in abortion, but its combination with mifepristone is less effective and takes longer (21), which completely matched our findings.
One limitation of the study was that some patients did not comply with taking four tablets concurrently, and others were excluded due to a history of certain disorders such as asthma, thromboembolism, malignancy, liver disease, or porphyria.
5.1. Conclusions
Combining letrozole with misoprostol is recommended for medical abortions due to its positive impact on achieving complete abortion and reducing the time required for abortion.