1. Background
About two years and a half have passed since the onset of the acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic, and as of May 29, 2022, over 526 million confirmed cases and over six million deaths have been reported globally (1). However, the real number of dead ones is far more than the above-reported figure, and around 15 million deaths is estimated to be occurred due to COVID-19 around the world (2). The problem has not been terminated to this point, and this pandemic provoked a second pandemic, the “long-haulers”, i.e., individuals presenting with post-COVID symptoms (3). Therefore, attention is gradually shifting toward the post-acute care of COVID-19 survivors (4-6), and a growing number of studies reported about patients who were affected by COVID-19 and unable to return to their baseline health state (7-9). Primarily, the term long COVID was used by Perego (as cited by Raveendran et al.) in social media to explain the persistence of symptoms during weeks or months after primary SARS-CoV-2 infection, irrespective of the viral status (10). Thereafter, different terms have been used to describe this condition, including post-acute COVID syndrome (PACS), chronic post-acute COVID, persistent post-COVID, and long COVID (11). The post-acute COVID-19 condition is characterized by a wide range of new, returning, or chronic health problems that present at least four weeks after being infected with SARS-CoV-2. In the subacute/ongoing COVID-19, symptoms appear 4 - 12 weeks after the onset of acute COVID-19, while in the chronic or post-COVID-19 syndrome, symptoms appear or persist beyond 12 weeks after the beginning of acute COVID-19 and are not attributable to other diagnoses (12). Persistent post-COVID symptoms (lasting more than 24 weeks) were also proposed by an integrative classification (3). In terms of clinical presentation, long COVID symptoms may overlap with other post-viral syndromes, such as muscle weakness, fatigue, and sleep disturbance (8, 13, 14). However, it has a heterogenous picture as neuropsychiatric, cardiovascular, hematologic, musculoskeletal, pulmonary, dermatologic, endocrine, renal, and gastrointestinal symptoms and also multisystem inflammatory syndrome in children (MIS-C) (5, 15, 16).
2. Objectives
In this study, we aimed to detect the incidence of long COVID syndrome (LCS), its physical and psychological aspects, and also its determinant factors in previously hospitalized patients.
3. Methods
3.1. Study Design and Setting
This retrospective cohort study was conducted on patients who had been admitted from February 2020 (the beginning of the COVID-19 epidemic in Iran) to November 2021 in the COVID-19 referral Ali-Asghar hospital in Shiraz, capital city of Fars province in southern Iran. Sample size was primarily calculated as 384 using Cochrane formula based on a presumed prevalence of 50% of LCS in hospitalized patients (17, 18), margin error of 5%, and a confidence interval of 95%. However, by considering gender, design effect of 2, and drop-out rate of 30%, the needed sample was estimated as 1997. For sampling, we extracted the list of all admitted patients in the above period from the electronic hospital information system (HIS). The list included 14,707 patients. After cleaning the data and excluding patients with missing data, patients that had been passed less than 90 days after discharging from the hospital, and dead patients, 13,610 patients remained for sampling. In the next step, we determined the proportion of sampling in each of the five epidemic waves (first wave: February 19, 2020 to May 20, 2020, second wave: May 21, 2020 to September 21, 2020, third wave: September 22, 2020 to January 18, 2021, fourth wave: January 19, 2021 to June 7, 2021, and the fifth wave: June 8, 2021 to November 29, 2021). Then, in each wave, samples were selected based on systematic random sampling method.
3.2. Data Collection and Quality Assurance
For gathering data, we used a checklist, which consisted of three parts: Pre-hospitalization, hospitalization, and post-hospitalization data. In the pre-hospitalization part, questions about demographic, socioeconomic, anthropometric, and clinical backgrounds, as well as smoking, alcohol, and substance-using behaviors, were included. In the hospitalization part, we gathered data about medications, admission to the intensive care unit (ICU), oxygen saturation (%SO2), and hemoglobin (Hb) levels in the blood in times of admission and discharging from hospital. In the post-hospitalization part, long COVID-19 symptoms, continuity of care by physicians, medications, and vaccination against COVID-19 were queried. We also defined whether another member of patients’ first-degree relatives had been admitted to hospital due to COVID-19 or not. Contents of this checklist was validated after several rounds of meetings among specialists, including two specialists in infectious diseases, a pulmonologist, an epidemiologist, a community medicine specialist, two internists, and two psychologists. Due to the concurrency of data gathering in this study with the COVID-19 epidemic and its strict lockdown condition, filling of checklists was accomplished by phone interview with each of the interviewees. Therefore, a trained female research assistant called each person and, after introduction of herself and an explanation about the study and its executives, requested them if they had consent to participate and answer the questions about pre- and post-hospitalization stages at the same call or another preferred time. In case of few interviewees who could not answer directly to the interviewer, their caregivers were asked to answer the questions. All selected individuals were included in this study except those who did not agree to participate in this study and also patients with Alzheimer’s disease. In terms of questions regarding hospitalization course, a team consisting of four trained general physicians extracted information from HIS and archived medical records in the hospital.
The quality of checklist’ filling was conducted by random checking of 50 filled checklists and comparing them with the master list of admitted patients and HIS. The quality of data entry was also assessed by random checking of 50 checklists and their corresponding data in the SPSS. The data were analyzed by two experts, and their findings were compared.
3.3. Statistical Methods and Data Analysis
All data of filled checklists were entered into the IBM® SPSS® statistics software version 25. Univariate analysis was done using an independent t-test and chi-square test. Then, as multivariate analysis, variables with P-value ≤ 0.2 were entered into the binary logistic and were analyzed by the backward wald method. Finally, variables with P-value < 0.05 were determined as the significant determinants of LCS.
3.4. Ethical Approval
The proposal of this study was approved by Ethics Committee affiliated with Shiraz University of Medical Sciences (SUMS), Shiraz, Iran, by No: IR.SUMS.REC.1400.295. Furthermore, we considered Helsinki ethical principles for medical research in this study. It should be mentioned that we provided for all interviewees, sufficient information about the purpose and process of our research as well as their rights, while verbal consent about voluntary participation in this study and permission for access to their hospital data was also obtained from each participant.
4. Results
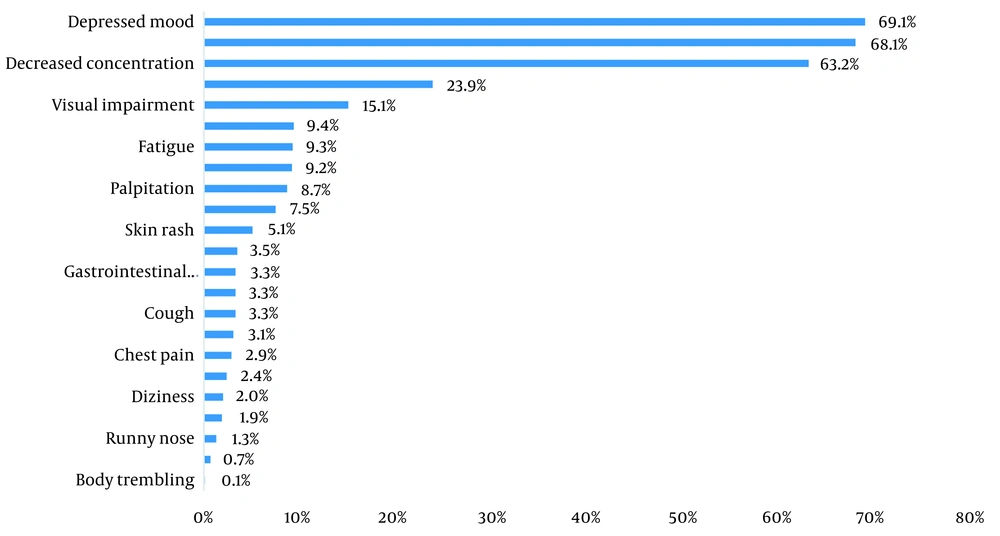
Participation rate in this study was 1738/1997; 87%, while a mean age of interviewees was 54.2 ± 14.5 years; 1,109 (63.8%) were female, and most of them (1,349; 77.6%) were educated up to 12 years, and married (1325; 76.2%). Being employed was reported by 1,000 (57.5%), while 765 (44%) were the head of their families, and 801 (46.1%) reported that their expenses were balanced with their incomes. In terms of background disease, 492 (28.3%) had hypertension (HTN), 152 (8.7%) had diabetes mellitus (DM), 106 (6.1%) had pulmonary diseases, such as asthma, 225 (12.9%) had cardiovascular diseases, 136 (7.8%) had anemia, and 167 (9.7%) had psychological diseases such as anxiety or depression. Furthermore, 123 (7.1%) were cigarette smokers, 38 (2.2%) had history of alcohol consumption, and 23 (1.3%) were substance users (Table 1). Median time of follow-up (period between attendance in this study and discharging from hospital) was 352 days. Among all participants, four (0.2%) had only pulmonary symptoms, 115 (6.5%) had only extra-pulmonary symptoms, 545 (31.4%) had only psychological symptoms, 862 (49.6%) had both physical and psychological symptoms, and 212 (12.2%) did not have any physical or psychological symptom. These figures show that 1,526 (87.8%) had LCS. In terms of prevalent symptoms, among pulmonary symptoms, dyspnea 130 (7.5%), among physical but extra-pulmonary symptoms, hair loss (415; 23.9%), and among psychological symptoms, feeling of unhappiness or depressed mood (1,201; 69.1%) were more common (Figure 1). Menstrual disorders were reported by 92 (8.3%) women. Out of all interviewees, 1,126 (64.7%) were referred to physicians after discharging from hospital, while 283 (16.3%) used antibiotics, 52 (3%) were on corticosteroids, 24 (1.4%) were on anticoagulants, and 941 (54.1%) used vitamins/mineral supplements. Furthermore, 1,075 (61.8%) received at least one dose of vaccine against COVID-19. According to the COVID-19 epidemic waves, 221 (11.4%), 467 (24%), 304 (15.7%), 501 (25.9%), and 434 (22.4%) were related to 1st to the 5th waves, respectively. Univariate analysis showed that job status, being the head of family, having a comorbidity, type of the COVID-19 epidemic wave, history of family members’ hospitalization due to COVID-19, being treated by antiviral or corticosteroid drugs during hospital admission, post-hospital care by physicians, and use of antibiotics or vitamins/minerals supplements after discharging from hospital correlated with LCS, with P-value ≤ 0.2 (Table 2). Multivariable analysis showed that anemia (odds ratio (OR): 3.22, 95% confidence interval (CI): 1.49 - 6.98), patients of the second epidemic wave (OR: 2.82, 95% CI: 1.57 - 5.07), use of vitamins/minerals supplements (OR: 2.25, 95% CI: 1.53 - 3.3), or antibiotics (OR:1.84, 95% CI: 1.02 - 3.33) after discharging from hospital, diabetes mellitus (OR: 1.9, 95% CI: 1.11 - 3.23), those who were not the head of their families (OR: 1.65, 95% CI: 1.18 - 2.32) and who were treated with antiviral drugs during hospital admission (OR: 1.64, 95% CI: 1.03 - 2.61) were significantly associated with LCS (Table 3).
| Variables | Values | Variables | Values |
|---|---|---|---|
| Age (y) | 54.26 ± 14.53 | ||
| Gender | Kidney disease | ||
| Male | 628 (36.1) | Yes | 76 (4.4) |
| Female | 1,109 (63.8) | No | 1,638 (94.2) |
| Education level (y) | Liver disease | ||
| ≤ 12 | 1,349 (77.6) | Yes | 56 (3.2) |
| > 12 | 360 (20.7) | No | 1,657 (95.3) |
| Marital status | Autoimmune disease | ||
| Single life | 370 (21.3) | Yes | 71 (4.1) |
| Married | 1,325 (76.2) | No | 1,639 (94.3) |
| Number of children (in married ones) | 3.53 ± 2.31 | ||
| Job status | Anemia | ||
| Employed | 1,000 (57.5) | Yes | 136 (7.8) |
| Non-employed | 711 (40.9) | No | 1,576 (90.7) |
| Being covered by insurance system | Cancer | ||
| Yes | 1,597 (93.1) | Yes | 25 (1.4) |
| No | 118 (6.8) | No | 1,689 (97.2) |
| Being the family financial decision maker | Neurological disease | ||
| Yes | 765 (44.0) | Yes | 37 (2.1) |
| No | 942 (54.2) | No | 1,675 (96.4) |
| Balanced income/cost | Psychological disease | ||
| Yes | 801 (46.1) | Yes | 167 (9.7) |
| No | 853 (49.1) | No | 1,543 (90.2) |
| Body mass index (kg/m2) before COVID-19 pandemic | 28.59 ± 5.96 | ||
| HTN | Corticosteroid drugs use | ||
| Yes | 492 (28.3) | Yes | 42 (2.4) |
| No | 1,224 (70.4) | No | 1,670 (96.1) |
| DM | Cigarette Smoking | ||
| Yes | 152 (8.7) | Yes | 123 (7.1) |
| No | 1,560 (90.0) | No | 1,605 (92.4) |
| Pulmonary disease | Alcohol consumption | ||
| Yes | 106 (6.1) | Yes | 38 (2.2) |
| No | 1,605 (92.4) | No | 1,687 (97.1) |
| Cardiovascular disease | Substance use | ||
| Yes | 225 (12.9) | Yes | 23 (1.3) |
| No | 1,489 (85.7) | No | 1,704 (98.0) |
Abbreviations: HTN, hypertension; DM, diabetes mellitus.
a Values are expressed as mean ± SD or No. (%).
| Variables | Long COVID - 19 Syndrome n= 1526 (87.8%) | Normal n = 212 (12.2%) | P - Value | OR | 95% CI OR |
|---|---|---|---|---|---|
| Age (y) | 54.18 ± 14.55 | 54.79 ± 14.44 | 0.574 | - | - |
| Gender | |||||
| Female/male | 975 (87.9)/550 (87.6) | 134 (12.1)/78 (12.4) | 0.836 | 1.03 | 0.76 - 1.39 |
| Education (y) | |||||
| ≤ 12/ > 12 | 1,198 (88.5)/309 (87) | 156 (11.5)/46 (13) | 0.456 | 1.14 | 0.8 - 1.62 |
| Marital status | |||||
| Single/married | 328 (88.6)/1161 (87.6) | 42 (11.4)/164 (12.4) | 0.593 | 1.1 | 0.77 - 1.58 |
| Number of children | 3.51 ± 2.29 | 3.69 ± 2.48 | 0.295 | - | - |
| Jobstatus | |||||
| Non-employed/employed | 883 (88.3)/403 (82.9) | 117 (11.7)/83 (17.1) | 0.004 | 1.55 | 1.14 - 2.10 |
| Being covered by insurance system | |||||
| No/yes | 100 (84.7)/1411 (88.4) | 18 (15.3)/186 (11.6) | 0.243 | 0.73 | 0.43 - 1.25 |
| Being the family financial decision maker | |||||
| No/yes | 839 (89.1)/665 (86.9) | 103 (10.9)/100 (13.1) | 0.175 | 1.22 | 0.6 - 1 |
| Balanced income/cost | |||||
| No/yes | 748 (87.7)/704 (87.9) | 105 (12.3)/97 (12.1) | 0.9 | 0.98 | 0.75 - 1.36 |
| BMI before being infected by COVID-19 (kg/m2) | 28.63 ± 6.13 | 28.63 ± 5.78 | 0.996 | - | - |
| BMI at the time of this study (kg/m2) | 28.23 ± 6.16 | 28.11 ± 5.46 | 0.813 | - | - |
| ΔBMI (kg/m2) | -0.37 ± 2.29 | -0.54 ± 1.92 | 0.375 | - | - |
| Background chronic disease | |||||
| No/yes | 595 (90.2)/915 (86.6) | 65 (9.8)/142 (13.4) | 0.026 | 1.42 | 1.04 - 1.93 |
| Smoking | |||||
| No/yes | 1,296 (88)/228 (89.4) | 177 (12)/27 (10.6) | 0.514 | 0.86 | 0.56 - 1.33 |
| Alcohol consumption | |||||
| No/yes | 1,480 (88.3)/41 (85.4) | 197 (11.7)/7 (14.6) | 0.549 | 1.28 | 0.56 - 2.89 |
| Substance use | |||||
| No/yes | 1,493 (88.3)/30 (83.3) | 198 (11.7)/6 (16.7) | 0.428 | 1.5 | 0.61 - 3.66 |
| COVID-19 epidemic waves | |||||
| 1st wave of epidemic | 202 (91.4) | 19 (8.6) | |||
| 2nd wave of epidemic | 356 (76.2) | 111 (23.8) | |||
| 3rd wave of epidemic | 265 (87.2) | 39 (12.8) | |||
| 4th wave of epidemic | 468 (93.4) | 33 (6.6) | |||
| 5th wave of epidemic | 223 (96.1) | 211 (3.9) | < 0.001 | - | - |
| ICU admission due to COVID-19 | |||||
| No/yes | 1,269 (87.9)/227 (87) | 175 (12.1)/34 (13) | 0.681 | 1.06 | 0.733 - 1.61 |
| History of family members d hospitalization due to COVID-19 | |||||
| No/yes | 1,098 (87.3)/391 (90.1) | 160 (12.7)/43 (9.9) | 0.12 | 0.755 | 0.529 - 1.077 |
| Antiviral drug use in hospital | |||||
| No/yes | 166 (82.2)/1360 (88.5) | 36 (17.8)/176 (11.5) | 0.009 | 0.597 | 0.403 - 0.884 |
| Corticosteroid use in hospital | |||||
| No/yes | 364 (84.7)/1162 (88.8) | 66 (15.3)/146 (11.2) | 0.021 | 0.693 | 0.506 - 0.948 |
| Antibiotic use in hospital | |||||
| No/yes | 118 (86.1)/1408 (87.9) | 19 (13.9)/193 (12.1) | 0.534 | 0.851 | 0.513 - 1.414 |
| Immunoglobulin use in hospital | |||||
| No/yes | 1,361 (88)/165 (85.9) | 185 (12)/27 (14.1) | 0.403 | 1.2 | 0.779 - 1.86 |
| SO1 (on hospital admission) | 89.07 ± 6.35 | 88.97 ± 6.3 | 0.841 | - | - |
| SO2 (on hospital discharge) | 92.95 ± 3.7 | 93.23 ± 3.45 | 0.314 | - | - |
| ΔSO2 | 3.94 ± 5.86 | 4.28 ± 5.65 | 0.434 | - | - |
| ΔHb (gr/dL) | -0.64 ± 3.2 | -0.57 ± 1.05 | 0.756 | - | - |
| Post-hospital discharge care by physicians | |||||
| No/yes | 527 (86.1)/999 (88.7) | 85 (13.9)/127 (11.3) | 0.112 | 0.78 | 0.58 - 1.05 |
| Post-hospital discharge corticosteroid use | |||||
| No/yes | 1,482 (87.9)/44 (84.6) | 204 (12.1)/8 (15.4) | 0.476 | 1.32 | 0.61 - 2.84 |
| Post-hospital discharge antibiotic use | |||||
| No/yes | 1,260 (86.6)/266 (94) | 195 (13.4)/17 (6) | 0.001 | 0.41 | 0.24 - 0.69 |
| Post-hospital discharge anticoagulants use | |||||
| No/yes | 1,504 (87.7)/22 (91.7) | 210 (12.3)/2 (8.3) | 0.759 | 0.65 | 0.15 - 2.78 |
| Post-hospital discharge vitamins/minerals use | |||||
| No/yes | 644 (80.8)/882 (93.7) | 153 (19.2)/59 (6.3) | < 0.001 | 0.282 | 0.2 - 0.38 |
| Being vaccinated against COVID-19 | |||||
| No/yes | 150 (89.3)/965 (89.8) | 18 (10.7)/110 (10.2) | 0.848 | 0.94 | 0.56 - 1.61 |
Abbreviations: OR, odds ratio; CI, confidence interval; BMI, body mass index; ΔBMI, delta body mass index (difference of body mass index between the times of this study and before of being infected by COVID-19); ICU, intensive care unit; SO1, blood level of oxygen saturation; ΔSO2, delta SO2 (difference of SO2 at the time of hospital admission and at the time of discharging from hospital); ΔHb (difference of blood level of hemoglobin at the time of hospital admission and at the time of discharging from hospital).
a Values are expressed as mean ± SD or No. (%) unless otherwise indicated.
| Variables | B | SE | P-Value | OR | 95% CI OR |
|---|---|---|---|---|---|
| Constant | 1.12 | 0.33 | 0.001 | 3.08 | - |
| Known case of anemia, yes/no (Ref) | 1.17 | 0.39 | 0.003 | 3.22 | 1.49 - 6.98 |
| COVID-19 epidemic wave (1st wave compared to the 2nd wave) | 1.04 | 0.29 | < 0.001 | 2.82 | 1.57 - 5.07 |
| History of vitamins/minerals use after discharge from admission to hospital due to COVID-19, yes/no (Ref) | 0.81 | 0.19 | < 0.001 | 2.25 | 1.53 - 3.3 |
| Known case of DM type 2, yes/no (Ref) | 0.64 | 0.27 | 0.018 | 1.9 | 1.11 - 3.23 |
| History of antibiotic use after discharging from admission to hospital due to COVID-19, yes/no (Ref) | 0.61 | 0.3 | 0.04 | 1.84 | 1.02 - 3.33 |
| Being the financial decision maker of family, no/yes (Ref) | 0.5 | 0.17 | 0.003 | 1.65 | 1.18 - 2.32 |
| History of antiviral drug treatment during hospital admission, yes/no (Ref) | 0.49 | 0.23 | 0.03 | 1.64 | 1.03 - 2.61 |
Abbreviations: B, beta; SE, standard error; OR, odds ratio; CI, confidence interval, Ref, reference; DM, diabetes mellitus.
5. Discussion
5.1. Key Findings
Long COVID syndrome, or post-acute COVID syndrome as the 2nd pandemic following 1st acute pandemic, has involved a large proportion of survived COVID-19 patients. In this study, it was revealed that more than four of five COVID-19 patients suffer from this syndrome with a wide variety of symptoms and organ involvement even after a median period of 352 days following discharging from hospital. We also found that four of five patients had at least one psychological symptom, while almost one of two patients had at least one physical symptom. Among pulmonary symptoms, dyspnea, and among extra-pulmonary symptoms, hair loss, and among psychological symptoms, depressed mood and feelings of unhappiness were more prevalent. Our results also revealed that anemic patients, patients who came up from the 2nd epidemic wave of COVID-19, those who had used vitamins/minerals supplements or antibiotics after discharging from hospital, diabetic patients, patients who were the head of their families, and who were treated with antiviral drugs during hospital admission due to COVID-19 were significantly involved more by LCS.
5.2. Interpretation of the Findings
In line with our study, a meta-analysis showed that 80% of the SARS-CoV-2 infected patients developed one or more long-term symptoms. This study found that these patients suffered more from fatigue (58%), headache (44%), attention disorder (27%), hair loss (25%), and dyspnea (24%) (19). Another meta-analysis revealed that the most commonly reported complaints among patients with LCS were fatigue (32%), dyspnea (25%), sleep disorder (24%), and difficulty in concentrating (22%), respectively, at 3 - 6 months follow-up. This study also revealed effort intolerance (45%), fatigue (36%), sleep disorder (29%), and dyspnea (25%) in patients, respectively, at 6 - 9-months follow-up. Fatigue (37%) and dyspnea (21%) were also reported at 9 - 12 months, and fatigue (41%), dyspnea (31%), sleep disorder (30%), and myalgia (22%) were claimed, respectively after 12-months follow-up (20).
A 6-month retrospective cohort study on 273,618 survivors of COVID-19 showed a mean age of 46.3 years among COVID-19 survivors, while 55.6% of them were female, 57% had one or more long-COVID feature during the first 6-month period, including 36.5% between three and six months. Abnormal breathing (18.7% in the first six-months period; 7.9% between 3 - 6-months period), fatigue (12.8%; 5.8%), chest/throat pain (12.6%; 5.7%), headache (8.6%; 4.6%), other pain (11.6%; 7.1%), abdominal symptoms (15.5%; 8.2%), myalgia (3.2%; 1.5%), cognitive symptoms (7.8%, 3.9%), and anxiety/depression (22.8%; 15.4%) were among noticed symptoms. Compared to influenza, all nine mentioned symptoms were more frequently reported after COVID-19 (with an overall excess incidence of 16.6% and hazard ratios 1.4 - 2, P < 0.001), and they also co-occurred more commonly. This study showed that significant differences in incidence and co-occurrence of LCS were also associated with gender, age, and severity of disease (21). Compared to our study, the incidence of LCS in this study was less than half of our finding (36.5% vs. 87.8%). However, reported symptoms such as dyspnea (7.9% vs. 7.5%), chest pain (5.7% vs. 2.9%), headache (4.6% vs. 3.1%), and abdominal symptoms (8.2% vs. 3.3%) were more prevalent in this study compared to fatigue (5.8% vs. 9.3%) and depression (15.4% vs. 69.1%), which were more prevalent in our study. Furthermore, and in contrast to the above study, we did not find any association between LCS and age or gender.
Another meta-analysis found that the Global estimated pooled prevalence of post-COVID-19 conditions was 43% (95% CI: 39 - 46%), including 54% (95% CI: 44 - 63%) in hospitalized and 34% (95% CI: 25 - 46%) in non-hospitalized patients. Regional estimated prevalence included Asia; 51% (95% CI: 37 - 65%), Europe; 44% (95% CI: 32 - 56%), and North America 31% (95% CI: 21 - 43%). Global prevalence for 30, 60, 90, and 120 days after infection were estimated to be 37% (95% CI: 26 - 49%), 25% (95% CI: 15 - 38%), 32% (95% CI: 14 - 57%) and 49% (95% CI: 40 - 59%), respectively. Fatigue was the most common symptom reported with a prevalence of 23% (95% CI: 17 - 30%), followed by memory problems (14%; 95% CI: 10 - 19%) (17). These figures show a less prevalence of LCS than what we found in our study (54% vs. 87.8%), while fatigue was more prevalent at global level compared to our findings (23% vs. 9.3%). Similarly, a prospective cohort study in Northwest Spain showed a more incidence of LCS among hospitalized versus non-hospitalized patients in a 6-month follow-up period (52.3% vs. 38.2%) (18).
A multicenter cohort study from the UK remarked that 93% of COVID-19 patients reported persistent symptoms and claimed that fatigue was the most common (83%), while breathlessness (54%), dyspnea (46%), and new or worsening disability (24%) were also common (14). According to some studies, the severity of the acute COVID-19 infection and baseline health status was associated with diverse symptoms in LCS (6, 22); however, different underlying mechanisms may have a role accordingly (3). We found an association between baseline anemia and DM or treatment with antiviral drugs (during admission to hospital) with LCS; however, level of SO2% or ICU admission did not correlate with LCS, which may show the severity of acute COVID-19 infection.
5.3. Strengths, Limitations, and Recommendations
As study strengths, we conducted this study on a large sample of patients who came up from different COVID-19 epidemic waves. We also assessed post-hospitalization medication, body mass index (BMI), vaccination against COVID-19, smoking, alcohol and substance use, and position of individual in the family. Furthermore, extraction of hospitalization data and quality checking were done by physicians, thereby reinforcing the accuracy of data gathering. In contrast, our findings could be more representative if a prospective cohort study with control group was possible to be conducted, if hospital records about the severity of acute COVID-19 infection were more informative, if males were more responsive, and if our information about background clinical diseases of patients was not only based on self-report but also relied on medical documentations. Furthermore, our findings about correlation of using antibiotics and/or vitamins and minerals with LCS in post-hospital discharging period need more confirmatory and prospective investigation to clarify whether they were prescribed as treatment of LCS or that LCS appeared as a side effect of them. We also recommend assessing knowledge and practice of COVID-19 patients about LCS since the importance of such studies may not be less than what was learned from studies in acute phase of COVID-19 pandemic (23).
Last but not least, “long COVID is devastating and debilitating for individuals - both young and old -, communities, and economies; thus governments need to take it seriously and provide integrated care, psychosocial support, and sick leave for those patients that are suffering from it” as Dr. Tedros Adhanom Ghebreyesus, WHO director-general, remarked at the WHO press conference on May 10, 2022 (2).
5.4. Conclusions
COVID-19 pandemic is not over and most patients with COVID-19 suffer from LCS during the first year after discharging from hospital, while there is no defined surveillance or care system for them. Therefore, as post-COVID-19 crisis management, making guidelines and establishment of integrated care system for these patients are highly needed and recommended.