1. Background
Congenital atrioventricular heart block (CAVB) occurs during the fetal period due to various etiological factors (1). Congenital complete heart block (CCHB) is a condition where the atrioventricular (AV) node is severely damaged, resulting in a slow heart rate of 40 to 80 beats per minute. This could be associated with cardiomegaly, heart failure, or even hydrops fetalis (2, 3). Although fetal death can be a devastating outcome, some fetuses successfully complete the prenatal period but may require permanent pacemaker (PPM) implantation after birth (4).
The estimated frequency of CAVB is one in 10,000 to 20,000 newborns. However, fetal involvement may be more common due to overlooked cases resulting from fetal death (4, 5). Maternal autoantibodies, including La (Sjogren-syndrome-related antigen B, SSB) and Ro (Sjogren-syndrome-related antigen A, SSA) antigen antibodies, are the most commonly reported etiological factors of CAVB, with or without symptomatic maternal systemic lupus erythematosus (SLE) (6). Ro and La antigen antibodies are the primary rheumatoid autoantibodies that can cross the placenta and affect the fetal heart (7). Additionally, certain fetal congenital heart anomalies, such as congenitally corrected transposition of the great arteries (CCTGA), may be linked to CAVB (8). Furthermore, the supposed risk factors for CAVB and CCHB include type 2 diabetes mellitus, hypothyroidism, maternal cytomegalovirus infection, and the use of antiepileptic medications (5).
The estimated prevalence of positive La/Ro antibodies among all pregnancies is approximately 2.8%. However, only a small percentage (2 - 5%) of this population will experience fetal CCHB (3, 9). However, in mothers with overt SLE, the incidence of fetal CCHB may increase up to 7%. Similarly, a higher titer of maternal autoantibodies (> 50 - 100 U/mL) is usually associated with a greater risk of CAVB (10). On the other hand, mothers who have experienced previous cases of fetal CCHB are at an even higher risk for the condition in their subsequent pregnancies, with a risk range of 15 to 21% (3, 9, 11). Transplacental transmission of inflammatory autoantibodies may result in scarring and fibrosis of the fetal conduction system. Atrioventricular block (AVB) is the most significant consequence of this inflammatory process (11). Similarly, the sinus node may be involved, resulting in subsequent sinus bradycardia. Likewise, inflammation of the conduction system at other levels may be associated with different types of arrhythmia, including atrial flutter or atrial bigeminy/trigeminy (1, 12). On the other hand, myo-endocardial inflammation may lead to various conditions, such as myocarditis, cardiomyopathy, atrioventricular valve regurgitation, and endocardial fibroelastosis (EFE) (1, 11). The critical period for the development of CAVB is between 16 and 24 weeks of gestational age, although earlier or later gestational ages may also have this vulnerability (13, 14). Also, CAVB typically starts with a first-degree AV block and may progress to the second or third-degree. Both types of second-degree AV block, including Mobitz 1 and 2, can occur in affected fetuses. Moreover, CCHB is characterized by atrioventricular dissociation, which results in a low ventricular rate (between 40 and 80 bpm), and typically a normal atrial rate (2, 12).
Fetuses with CCHB are at an increased risk of prenatal death, particularly those with a ventricular rate of less than 55 bpm, left ventricular dysfunction, atrioventricular valve dysfunction, heart failure, endocardial fibroelastosis, hydrops fetalis, development of CCHB before 20 weeks of gestational age, and higher levels of serum autoantibodies (> 50 - 100 U/mL) (11). Preventive medications, such as steroids and hydroxychloroquine (HCQ), may show promise in the early diagnosis of first or second-degree AVB (5). Corticosteroids, especially fluorinated types, were administered as the mainstay of therapy with discouraging results (15). Recently, HCQ has been reported to have optimistic effects (16). On the other hand, administration of IVIG and/or plasmapheresis has shown encouraging results in pregnancies with positive autoantibodies (17). No preventive medication is typically effective once CCHB is established. However, prompt heart failure or hydrops fetalis management is critical (3). Additionally, some reports have recommended steroid administration during the acute phase of CCHB (9).
2. Objectives
This survey aims to present the results of our predictive and therapeutic protocols at a fetal heart center. This will enable the introduction of the best therapeutic methods based on further studies in the future.
3. Methods
The diagnostic and management strategies were implemented through close collaboration between the departments of pediatric cardiology and perinatology. We admitted 15 pregnant mothers with varying degrees of CAVB at the Kurdistan University Fetal Heart Center between November 2007 and August 2022. Our cases were referred by primatologists, gynecologists/obstetricians, sonographers, and rheumatologists before or after the diagnosis of CCHB was established. Some of them were also diagnosed in our clinic by detecting a high PR interval. After obtaining a complete medical history, we conducted fetal echocardiography and ordered testing for maternal Lo/Ra autoantibodies. Echocardiography is the standard diagnostic tool for various types of CAVB. In this study, Doppler and M-mode echocardiography were the primary methods used for early diagnosis of CAVB, utilizing the Samsung HS70A apparatus manufactured in Korea. The first-degree AVB is highly suggestive if the PR interval exceeds 150 ms and diagnostic if values exceed 160 ms, as indicated by Doppler mode (9, 11, 18). Likewise, both types of second-degree AVB can be identified using Doppler mode. Third-degree AVB or CHB is easily indicated by both Doppler and M modes (5, 12). The main antigens involved in antibodies are La (Sjogren-syndrome-related antigen B, SSB) and Ro (Sjogren-syndrome-related antigen A, SSA). A titer of 8 - 49 U/mL is considered low positive, while titers exceeding 50 U/mL and 100 U/mL are considered moderate and high, respectively (10).
The management of immune-mediated second or third-degree AVB is relatively well-known and involves the early administration of appropriate medication. The therapeutic approach for second-degree AVB involved using dexamethasone and HCQ alone or in combination with IVIG. This treatment was recommended for cases with one or more of the following risk factors: Higher titer antibodies (> 50 - 100 U/mL), a previous history of CCHB, or a previous history of fetal death. Dexamethasone is recommended for short-term use of 2-4 weeks at a dosage of 4 - 8 mg daily due to potential side effects for both the mother and fetus (3, 5). Some studies have reported that short-term administration of HCQ (200 mg BD for two months) may be effective in treating new cases of second-degree AVB (3, 14, 19). However, the administration of this preventive course is long-term, beginning at approximately 10 weeks of gestational age and continuing until the end of the pregnancy (16, 19). The recommended course of IVIG is 1 g/kg, administered four times at a two-week interval (3, 19). In CCHB, treatment is focused on managing bradycardia and/or heart failure. Our case series followed the recommended dosages and treatment durations for the aforementioned medications.
Currently, the management of immune-mediated first-degree AVB is unclear. Our therapeutic strategy for these cases is interconnected with the presence of major or minor risk factors:
(A) Major risk factors of CCHB include previous history of CCHB, moderate or high titers of SSB/A antibodies (> 50 - 100 U/mL), previous history of fetal death, mild to moderate bradycardia (HR < 110 bpm), constantly high PR interval (> 165 ms), progressive increase in PR interval, and early evidence of myocarditis (such as cardiomegaly, atrioventricular regurgitation, and endocardial echo-densities) (3, 9, 11).
(B) Minor risk factors for CCHB include type 2 diabetes mellitus, hypothyroidism, and the use of antiepileptic drugs (5).
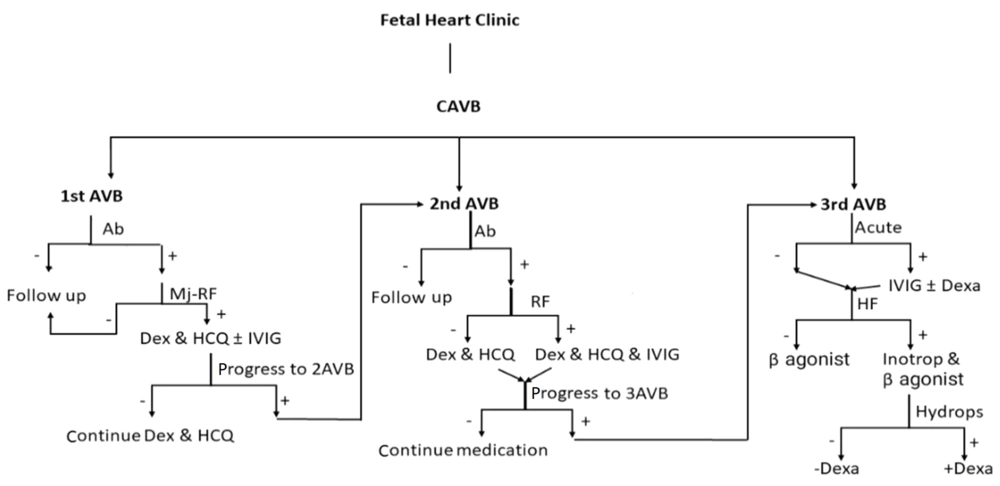
Early medication for first-degree AVB should be carefully considered in light of its potential self-limiting nature and the risk of drug side effects. In our protocol, the presence of a major risk factor for immune-mediated first-degree AVB suggests starting medication. Similarly, the presence of any minor risk factors warrants close follow-up through fetal echocardiography. Therefore, we opted for a weekly echocardiographic assessment during the critical period of gestational age (16 to 25 weeks) for high-risk pregnancies (Figure 1).
4. Results
In our study, the gestational age at the first visit ranged from 14 to 32 weeks, with a mean of 21.7 weeks. Nine fetuses (60%) had third-degree AVB, while four and three cases had first-degree and second-degree AVB, respectively. During the initial visit, the fetal heart rates ranged from a minimum of 47 to a maximum of 145. Of the 86% of cases with bradycardia, 53% had CCHB and a heart rate of less than 60 bpm, while the remaining 33% had first- and second-degree AVB with heart rates between 75 and 110 bpm. Four cases were referred to as fetal arrhythmia, with a final diagnosis of either second-degree AVB or mixed second/third-degree AVB (Table 1).
| Case Number | Age of Mother | G/P | Sex | GA1 | GA2 | HR1 | HR2 | Block1 | Block2 | SSA/B, U/ML | CHD | MED |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 29 | 1/1 | M | 22 | 26 | 140 | 134 | G -1 (155) | G – 1 (155) | < 8 | - | NO |
| 2 | 35 | 6/1 | F | 17 | 27 | 107 | 112 | G – 1 (185) | G – 1 (160) | 25 - 49 | - | CORT--HCQ |
| 3 | 24 | 1/1 | M | 21 | 23 | 110 | 58 | G - 2 | G - 3 | > 50 | - | CORT-HCQ-IVIG |
| 4 | 31 | 2/2 | F | 21 | 23 | 54 | 54 | G - 3 | G- 3 | 100 < SLE | - | CORT- SALB |
| 5 | 32 | 2/1 | F | 22 | 24 | 48 | 49 | G - 3 | G -3 | 100 < | - | CORT-SALB |
| 6 | 30 | 3/1 | F | 32 | 37 | 55 | - | G - 3 | G - 3 | 100 < | - | NO |
| 7 | 27 | 2/1 | M | 26 | 30 | 47 | - | G - 3 | G - 3 | < 8 | - | CORT- SALB |
| 8 | 25 | 1/1 | M | 22 | 26 | 68-133 | 75 | G -2&3 | G - 2 | > 100 | - | CORT-HCQ-IVIG |
| 9 | 22 | 1/1 | F | 30 | 34 | 57 | - | G - 3 | G - 3 | > 50 | - | NO |
| 10 | 31 | 2/1 | F | 17 | 21 | 102 | 90 | G -1 (170) | G – 1 (150) | > 50 | - | CORT-HCQ-IVIG |
| 11 | 27 | 2/1 | M | 22 | 26 | 53 | - | G - 3 | G - 3 | > 100 | - | CORT- SALB |
| 12 | 31 | 1/1 | M | 14 | - | 55 | - | G-3 | - | < 8 | CAVCD | NO |
| 13 | 36 | 3/1 | M | 20 | - | 48 | 48 | G - 3 | - | > 100 | - | SALB-DIG |
| 14 | 26 | 2/1 | F | 18 | 25 | 145 | 148 | G-1 (165) | G-1 (155) | > 100 | - | CORT-HCQ--IVIG |
| 15 | 26 | 1/1 | M | 22 | 26 | 90 | 130 | G-2 | NSR | > 100 | _ | CORT-HCQ-IVIG |
Abbreviations: G/P, gravidity and parity; GA, gestational age at two randomly selected of echo examinations; HR, heart rate at two times of echo examination; Block, type of block at two times of echo examinations; G, grade of block; PR interval by ms; SSA/B, Sjogren-syndrome-related antigen A and B; CHD, congenital heart disease; CAVCD, complete atrioventricular canal defect; MED, medication; CORT, corticosteroid = dexamethasone; HCQ, hydroxychloroquine; IVIG, intra venus immune globulin; SALB, salbutamol; DIG, digoxin; NSR, normal sinus rhythm; + SLE, positive history of maternal systemic lupus erythematosus.
Ro and La antibody titers were positive in 12 out of 15 cases (80%), despite only one patient having overt lupus disease. A previous maternal history of abortion or fetal death was present in seven out of fifteen cases, accounting for 47% of the total cases. Overt type 2 diabetes mellitus and hypothyroidism were detected in six (40%) and four (27%) cases, respectively.
All fetuses with CCHB exhibited varying degrees of cardiomegaly, but only two showed reduced LVEF and signs of hydrops fetalis. Two cases were referred by sonographers as hydrops fetalis but were later diagnosed as CCHB and confirmed through positive autoantibody tests. We did not find any fetus with cardiac echo density indicating EFE.
Among the four cases with first-degree AVB (PR interval greater than 155 to 160 ms), two fetuses (at gestational ages of 22 and 17 weeks) had negative and low autoantibody titers, respectively. These cases were monitored weekly during the first month. We began administering medication (dexamethasone and HCQ) for the second case, which had a low antibody titer, a very high PR interval, and a maternal history of five abortions. Fortunately, none of them experienced progressive AVB during or after pregnancy. On the other hand, out of two cases with moderate and high antibody titers, the first case (with a gestational age of 17 weeks) presented with first-degree AVB and constant sinus bradycardia (ranging from 90 to 102 bpm). This case underwent similar dual medication, which improved heart rate and a sequential decrease in PR intervals. The second case, at 18 weeks of gestational age, had a history of one previous abortion and a high titer of SSA antibodies. The patient was treated with a combination of dexamethasone (for one month), HCQ (throughout the entire pregnancy), and a full course of IVIG. Fortunately, there was no progression of AVB during or after pregnancy.
In a 22-week-old fetus with mixed second/third-degree AVB, IVIG was added to the combined medication of HCQ and dexamethasone, resulting in a successful permanent return to second-degree AVB. Two other cases, who were in their first pregnancy and had a gestational age of 21 and 22 weeks, respectively, were diagnosed with second-degree AVB and had positive autoantibody titers. Combined dexamethasone, HCQ, and a full course of IVIG were administered to both patients. Unfortunately, the first case, which initially presented with mild cardiomegaly and bradycardia (HR of 110 bpm), progressed to CCHB within one week of starting therapy. In contrast, the second case, which had a high titer of autoantibodies, returned to normal sinus rhythm. This suggests greater concern for first-time mothers with no history of abortion as a potential risk factor.
Two cases of CCHB referred with hydrops fetalis exhibited significant cardiomegaly and hemodynamic dysfunction, which were associated with high-titer antibodies. Both fetuses died after a few weeks of gestation despite administering digoxin and salbutamol. Of the eight cases admitted with CCHB, five had a history of previous abortion or a high titer of antibodies (62%) (Table 2).
| Case Number | DM | T4 | CCHB/p | CT/R | EF | Hydrops | Hx/Abort | Outcome | PPM-I |
|---|---|---|---|---|---|---|---|---|---|
| 1 | - | - | - | OK | OK | - | - | Good | - |
| 2 | + | - | - | OK | OK | - | 5 | Good | - |
| 3 | - | + | - | High | OK | - | - | CHB | - |
| 4 | - | - | - | High | OK | - | - | CHB | - |
| 5 | + | - | - | High | OK | - | 1 | CHB | - |
| 6 | - | - | - | High | OK | - | 2 | CHB | - |
| 7 | + | + | - | High | OK | - | 1 | CHB | - |
| 8 | - | - | - | OK | OK | - | - | G-2 | - |
| 9 | - | - | - | High | OK | - | - | CHB | - |
| 10 | + | - | + | OK | OK | - | - | Good | - |
| 11 | - | - | - | High | OK | - | 1 | CHB | - |
| 12 | - | - | - | High | 30% | + | - | Died | - |
| 13 | + | + | - | High | 35% | + | 2 | Died | - |
| 14 | - | + | - | OK | OK | - | 1 | Good | - |
| 15 | + | - | - | OK | OK | - | - | Good | - |
Abbreviations: DM, diabetes mellitus type 2; T4, hypothyroidism; CCHB/p, previous congenital complete heart block; CT/R, cardio thoracic ratio; high CT/R, > 0.35 area; EF, left ventricular ejection fraction; Hx/abort, history of abortion in mother; PPM-I, permanent pace maker implementation. Died, died after birth.
5. Discussion
There is no consensus on the therapeutic approach for first-degree AVB caused by immune-mediated factors (3-5). The main purpose of this study is to facilitate early diagnosis and treatment of suspected CAVB. We utilized the aforementioned major risk factors as either a basis for early echocardiographic evaluation or as a therapeutic guideline in cases of immune-mediated first-degree AVB. Our major risk factors included a history of CCHB, a history of fetal death, higher titers of SSB/A antibodies (> 50 - 100 U/mL), bradycardia (HR < 110 bpm), a high PR interval (> 165 ms) or progressive increase in PR interval, and early evidence of myocarditis (3, 9, 11). In our case series, only one pregnant mother had a previous history of CCHB. However, previous fetal death was a common occurrence, with a prevalence of 47%. This is the foreseeable consequence of the threatening nature of CCHB (20). Additionally, a previous history of fetal death is a risk factor for pregnant mothers who test positive for antibodies (20). Moderate and high antibody titers were present in 80% of our cases. However, high values (> 100 U/mL) were consistently observed in CCHB. This may indicate that higher titers are an important risk factor. A higher titer of SSB/SSA antibodies is typically associated with an increased risk of developing CCHB (3, 9). However, our results contradict this finding.
Various degrees of bradycardia, particularly those below 110 bpm, may be considered a potential predictor of CAVB (21). Constant bradycardia ranging from 90 to 110 bpm may be caused by inflammatory dysfunction of the sinus node (1, 2). However, more severe bradycardia, with a heart rate of less than 90 bpm, may be caused by second or third-degree AVB (22). Bradycardia was a common finding (86%) among our cases, including those with CCHB, second-degree AVB, and even first-degree AVB. Mild bradycardia (90 - 110 bpm) was detected in five cases (33%) of our study, both in first and second-degree AVB. Because bradycardia is not typically a direct result of first-degree AVB, it may be linked to other mechanisms, such as sinus node dysfunction (1, 12). Irregular fetal heart rate detected by sonography or ultrasonography could be the primary indicator of CAVB. This can be caused by second-degree AVB or atrial flutter (2, 22). Fetal cardiomegaly detected by ultrasonography may be due to myocarditis or CHB with extreme bradycardia (11, 23). In our case series, a fetus with second-degree AVB and mild cardiomegaly showed signs of possible early myocarditis. Endocardial fibroelastosis (EFE) is a concerning indication of CCHB, which is identified by sonographic echo density in various areas of the endocardium (11). Hydrops fetalis may result from heart failure during CCHB and has a poor prognosis (6, 7). Rapid diagnosis and management of CAVB depend on a regular protocol for early referral of pregnant mothers at around 16 weeks of gestation.
Likewise, inflammation of other parts of the conduction system can lead to various types of arrhythmia, such as atrial flutter or atrial bigemini/trigemini (1, 11). The severity of inflammation may also be linked to the specific type of arrhythmia observed (3, 5). In our experience, regularly measuring PR intervals in all fetuses can predict the early stages of CAVB. Unfortunately, our survey revealed that the referral times for cases of CCHB were between 21 and 22 weeks of gestational age. This confirms the characteristic rapid development of CCHB and the necessity for emergent screening of high-risk pregnancies (4).
Despite the low frequency of CCHB in pregnant mothers, certain risk factors can increase the likelihood of its development (24, 25). Type 2 diabetes mellitus and hypothyroidism may increase the risk of developing CAVB in cases of immune-mediated AVB (5). Additionally, administering medications such as antiepileptic drugs to pregnant women who have tested positive for autoantibodies may elevate the risk (26, 27). In our case series, we found that minor risk factors such as diabetes mellitus and hypothyroidism were relatively common. These factors served as a guideline for closely monitoring the cases with immune-mediated first-degree AVB through echocardiographic follow-up.
Most of our cases had positive autoantibody titers; however, only one had overt maternal lupus erythematosus. This may be related to early management of known cases of lupus erythematosus, mostly prior to pregnancy. Some studies have reported similar results (3, 11).
Starting medication for cases with first-degree AVB is controversial due to the debate surrounding their necessity versus their potential side effects (3, 14). Our therapeutic strategy for immune-mediated first-degree AVB depends on the major risk factors.
Currently, there is no established therapeutic protocol to prevent CCHB. However, corticosteroids have been considered the primary treatment option, although their effectiveness is not definitive. Typically, they are prescribed for a period of 2 to 4 weeks in cases of immune-mediated second-degree AVB. Additionally, some centers recommend early initiation after the onset of immune-mediated CCHB (3, 15). Dexamethasone is our primary medication for all cases of high-risk immune-mediated first-degree AVB and immune-mediated second-degree AVB. It is typically used in combination with HCQ. Unfortunately, we did not administer CCHB at the early stages of emergent treatment with this medication.
Moreover, IVIG, plasmapheresis, and HCQ have been used by many centers with relatively satisfactory outcomes (5, 15). Most references recommend administering these medications for second-degree AVB, especially in pregnancies with a positive autoantibody titer (3, 5). Some reports recommend administering IVIG during the early stages (12 - 24 hours after onset) of immune-mediated CCHB (3, 16, 19). Some reports recommend early prophylactic medication with IVIG and/or plasmapheresis for pregnancies with a previous history of CCHB and moderate or high positive autoantibody titers (10), which has shown satisfactory results. In our survey, IVIG was only used in high-risk cases of immune-mediated second-degree AVB, yielding satisfactory results. There is controversy surrounding the therapeutic or preventive role of HCQ. Some reports recommend using HCQ for pregnant mothers with a previous history of immune-mediated AVB, starting from early gestational ages (around 10 weeks) until its termination. However, some other centers use it to treat newly recognized cases of immune-mediated AVB, typically for shorter periods of around two months (3, 19). We administered long-term HCQ medication to treat immune-mediated first-degree AVB in patients with a history of CCHB or fetal death (Figure 1).
The main strategy for CCHB was the treatment of heart failure. In fetuses under 28 weeks of gestational age with CCHB (without congenital heart disease and hydrops fetalis), we attempted a short-term (2 to 4 weeks) administration of dexamethasone, but it did not produce the desired effects. Additionally, we started administering salbutamol (2.5 mg / 8 h) to patients with heart rates below 60 bpm, hoping to increase their heart rate. Saxena et al. recommended using beta-2 agonists such as terbutaline, salbutamol, or ritodrine to prevent further ventricular rate reduction, especially in cases where the rate is less than 60 bpm (19). Additionally, we have initiated digoxin treatment in CCHB cases complicated by heart failure. Some reports recommend using digoxin in cases of CCHB and heart failure. Some reports recommend the prompt administration of corticosteroids and/or IVIG for the recent onset of CCHB within 12 - 24 hours of its onset (4, 14). IVIG was not indicated in any of our cases of CCHB. None of the fetuses in our study required emergent pacemaker implantation at birth. However, medication for heart failure was used during the years following birth. Recently, we referred a 6-year-old with CCHB for PPM (permanent pacemaker implantation).
The main limitation of the study is the small sample size, which is attributed to the study being conducted in a single setting. The lack of randomization in using the protocol is another limitation. However, due to the small number of fetuses with CHB, these limitations will be evident in each single-center study.
5.1. Conclusions
Utilizing "Major and Minor Risk Factors" can aid in achieving our preventive objectives for immune-mediated congenital AVB. However, this protocol is a rudimentary guideline. For greater certainty, a larger population should be studied in the future.