1. Background
Functional gastrointestinal (GI) disorders encompass a range of conditions related to the digestive system that manifest due to disrupted communication between the brain and the intestines, leading to motility disturbances and increased GI tract sensitivity (1). Examples of such disorders include functional dyspepsia (FD) and irritable bowel syndrome (IBS), which collectively impact 16 - 26% of the global population (2, 3). The absence of clear diagnostic markers and an uncertain etiology hinder effective treatment options for these conditions (4).
While most intestinal infections are temporary, 5 - 30% of patients develop new, post-infectious GI symptoms even after the initial pathogen has been cleared. The advent of COVID-19, frequently associated with GI manifestations like diarrhea, abdominal pain, and nausea or vomiting (5-8), has spurred research interest in the development of post-infectious GI symptoms. Similar to other infections that precipitate acute GI symptoms, COVID-19 can result in lasting conditions such as post-infectious IBS and post-viral gastroparesis (9).
Previous studies have indicated that post-infectious IBS, a functional GI disorder, occurs in about 10% of individuals following acute infectious gastroenteritis (10). Moreover, the prevalence of GI symptoms among COVID-19 patients has been reported to range from 5 - 50% (11). Some researchers argue that the management of COVID-19 patients should extend beyond the acute phase of the disease to address potential chronic somatic conditions, including functional GI disorders (12, 13). With millions worldwide infected by COVID-19, even a small proportion experiencing persistent GI symptoms could pose a significant public health challenge.
2. Objectives
This study aimed to assess the frequency, severity, and risk factors associated with the development of functional disorders like GI symptoms, FD, and IBS one year post-COVID-19 infection.
3. Methods
3.1. Study Design and Participants
This cross-sectional study focused on patients with COVID-19 who visited Al-Zahra Hospital in Isfahan in 2021. With a 95% confidence level, an 80% test power, and based on previous research indicating a 15% incidence rate of IBS post-COVID-19 (14), along with an error margin of 0.06, the sample size was determined to be 400 individuals.
3.2. Inclusion and Exclusion Criteria
Eligible participants were those over 18 years old who had recovered from COVID-19 for one year. Exclusion criteria included patients with a history of GI disorders, chronic GI symptoms, IBD, IBS, FD, or cancer.
Upon securing ethical approval from the Ethics Committee of Isfahan University of Medical Sciences (approval code: IR.MUI.MED.REC.1400.478), access was obtained to the patient records at Al-Zahra Hospital. The list of COVID-19 patients hospitalized in the last 6 to 12 months was reviewed. Four hundred patients meeting the study criteria, with no prior GI disorders or underlying diseases related to the study's exclusion criteria, were selected through simple random sampling. These patients were contacted by phone to obtain their consent for participation, which was duly recorded. Any missing information on prior underlying diseases in the patients' files was clarified during this contact. Information such as demographic details (age, sex, occupation, education level), antibiotic usage during treatment, duration of hospital stay, and ICU admissions were extracted from the patient files.
One year post-COVID-19 infection, the patients were contacted again and asked to visit the hospital for a clinical assessment (evaluation of GI symptoms by a gastroenterologist) and to complete the ROME-III and Hospital Anxiety and Depression Scale (HADS) questionnaires.
Patients who passed away within one year post-infection or failed to attend the hospital for the clinical assessments were excluded from the study.
3.3. ROME-III Questionnaire
The Persian version of the ROME-III questionnaire was utilized to assess functional gastrointestinal disorders (FGIDs). This questionnaire, alongside patients’ clinical histories of IBS and its type, either IBS with predominant constipation (IBS-C) or predominant diarrhea (IBS-D), FD, and GI symptoms, such as vomiting, nausea, burping, diarrhea, constipation, bloating, and abdominal pain, was used for evaluation and recording. Reisswitz et al. stated that the Rome III Diagnostic Questionnaire for FD is a reliable instrument with a Cronbach’s alpha coefficient of 0.79 (15). Mahmoudzadeh et al.'s study in Iran showed that the content validity index (CVI) and content validity ratio (CVR) of this questionnaire ranged from 0.84 - 1 and exceeded 0.62, respectively. Furthermore, the Cronbach's alpha value ranged from 0.90 - 1. Factor analysis employing the Kaiser Meyer Olkin test (KMO) supported its validity by extracting a one-factor solution (KMO > 0.6; P < 0.05) (16).
3.4. The Hospital Anxiety and Depression Scale
The Hospital Anxiety and Depression Scale (HADS), a 14-item questionnaire, comprises 7 items each for depression and anxiety sub-scales. The total score ranges from 0 to 21, with higher scores indicating more severe anxiety and depressive disorders. Scores below 11 were considered indicative of the absence of anxiety or depression, while scores of 11 or above were considered to denote the presence of anxiety or depression. The reliability and validity of this questionnaire have been corroborated by prior research (17, 18).
3.5. Statistical Analysis
The compiled data was analyzed using SPSS software (version 26), presented as mean ± standard deviation (SD) or frequency (%). Logistic regression with the enter method was employed to examine the impact of the patient's demographic and clinical variables on the odds ratio of IBS, FD, and GI, with the odds ratio (OR) reported accordingly. A significance level of less than 0.05 was set for all analyses.
3.6. Ethical Consideration
The authors fully adhered to ethical standards, including avoidance of plagiarism, data fabrication, and duplicate publication. The research was conducted under the ethical approval code IR.MUI.MED.REC.1400.478 from the Ethics Committee of the respective university.
4. Results
Of the 400 patients eligible after one year of recovering from COVID-19, 43 were excluded due to either not attending the clinical examination or death, leaving 357 participants for the study. Of these, 49% were male, and 51% were female, with an average age of 48.79 ± 10.69 years. One year post-COVID-19 recovery, 55 patients (15.4%) developed IBS, including 4.8% with IBS-C and 10.6% with IBS-D. Gastrointestinal symptoms were observed in 160 cases (44.9%), with diarrhea, nausea, vomiting, and abdominal pain reported at 19%, 16%, and 15.7%, respectively. FD was identified in 34 cases (9.5%), and 2.8% had both FD and IBS (FD-IBS) (Table 1).
| Variables | Values a |
|---|---|
| Age, y | 48.79 ± 10.69 |
| Sex | |
| Male | 175 (49) |
| Female | 182 (51) |
| Job status | |
| Unemployed | 21 (5.9) |
| Freelance employment | 94 (26.3) |
| Employed | 110 (30.8) |
| Housewife | 118 (33.1) |
| Retired | 14 (3.9) |
| Level of education | |
| Illiterate | 36 (10.1) |
| Under diploma/diploma | 126 (35.3) |
| University education | 195 (54.6) |
| Received antibiotics | 301 (84.3) |
| ICU admission | 23 (6.4) |
| Length of hospital stay, day | 7.76 ± 0.32 |
| IBS | 55 (15.4) |
| IBS-C | 17 (4.8) |
| IBS-D | 38 (10.6) |
| GIsymptoms | 160 (44.9) |
| Diarrhea | 68 (19) |
| Constipation | 40 (11.2) |
| Abdominal pain | 56 (15.7) |
| Belching | 30 (8.4) |
| Bloating | 26 (7.3) |
| Nausea and vomiting | 57 (16) |
| FD | 34 (9.5) |
| FD-IBS | 10 (2.8) |
| Anxiety (HADS) | 234 (65.5) |
| Depression (HADS) | 128 (35.9) |
Basic and Clinical Information of the Patients
Analysis of factors influencing the onset of IBS showed that being female (OR (95% CI): 2.171 (1.181 - 3.989; P-value = 0.013)), having anxiety (OR (95% CI): 1.910 (1.230 - 4.942); P-value = 0.039), and depression (OR (95% CI): 1.772 (1.010 - 3.207); P-value = 0.040) significantly increased the odds of developing IBS. Conversely, freelancers and housewives had the lowest odds of developing IBS (OR: 0.285, 0.246; P-value < 0.05) (Table 2).
| Variables | IBS (n = 55) | Non-IBS (n = 302) | OR (95 CI) | P - Value | |
|---|---|---|---|---|---|
| IBS - C (n = 17) | IBS - D (n = 38) | ||||
| Age (y) | 47.53 ± 10.44 | 50.11 ± 14.48 | 48.77 ± 10.16 | 1.010 (0.979 - 1.042) | 0.524 |
| Sex | 0.013 | ||||
| Male | 5 (29.4) | 14 (36.8) | 156 (51.7) | Reference | |
| Female | 12 (70.6) | 24 (63.2) | 146 (48.3) | 2.171 (1.181 - 3.989) | |
| Job status | |||||
| Unemployed | 4 (23.5) | 2 (5.3) | 15 (5.0) | Reference | |
| Freelance employment | 4 (23.5) | 6 (15.8) | 84 (27.8) | 0.285 (0.087 - 0.931) | 0.038 |
| Employed | 4 (23.5) | 12 (31.6) | 93 (30.8) | 0.354 (0.114 - 1.103) | 0.073 |
| Housewife | 5 (29.4) | 17 (44.7) | 97 (32.1) | 0.246 (0.071 - 0.854) | 0.027 |
| Retired | 0 (0) | 1 (2.6) | 13 (4.3) | 0.309 (0.031 - 3.032) | 0.313 |
| Level of education | |||||
| Illiterate | 1 (5.9) | 3 (7.9) | 32 (10.6) | Reference | |
| Under diploma/diploma | 4 (23.5) | 14 (36.8) | 106 (35.1) | 1.863 (0.575 - 6.039) | 0.300 |
| University education | 12 (70.6) | 21 (55.3) | 164 (54.3) | 2.128 (0.643 - 7.049) | 0.216 |
| Received antibiotics | 10 (58.8) | 21 (55.3) | 270 (89.4) | 0.963 (0.301 - 3.073) | 0.863 |
| ICU admission | 2 (11.7) | 4 (10.5) | 4 (1.3) | 0.110 (0.030 - 0.403) | 0.700 |
| Length of hospital stay, day | 7.36 ± 10.69 | 6.73 ± 9.75 | 7.45 ± 6.13 | 1.011 (0.896 - 2.002) | 0.168 |
| Anxiety (HADS) | 12 (70.6) | 26 (68.4) | 198 (65.6) | 1.910 (1.230 - 4.942) | 0.039 |
| Depression (HADS) | 7 (41.2) | 20 (52.6) | 103 (34.1) | 1.772 (1.010 - 3.207) | 0.040 |
Risk Factors Associated with Development of IBS after COVID-19 a
Regarding GI symptoms, being female (OR (95% CI): 1.824 (1.01 - 3.394); P-value = 0.048) and having anxiety (OR (95% CI): 2.225 (1.426 - 3.470); P-value < 0.001) were associated with an increased odds ratio. Housewives had the lowest odds of developing GI symptoms (OR: 0.288 (0.095 - 0.872); P-value = 0.028) (Table 3).
| Variables | GI Symptoms (n = 160) | Any Symptoms (n = 197) | OR (95 CI) | P-Value |
|---|---|---|---|---|
| Age; y | 48.92 ± 11.39 | 48.69 ± 10.11 | 1.006 (0.980 - 1.032) | 0.664 |
| Sex | ||||
| Male | 76 (47.5) | 99 (50.3) | Reference | |
| Female | 84 (52.5) | 98 (49.7) | 1.824 (1.01 - 3.394) | 0.044 |
| Job status | ||||
| Unemployed | 12 (7.5) | 9 (4.6) | Reference | |
| Freelance employment | 37 (23.1) | 57 (28.9) | 0.450 (0.167 - 1.213) | 0.114 |
| Employed | 57 (35.6) | 53 (26.9) | 0.743 (0.278 - 1.986) | 0.554 |
| Housewife | 45 (28.1) | 73 (37.1) | 0.288 (0.095 - 0.872) | 0.028 |
| Retired | 9 (5.6) | 5 (2.5) | 1.445 (0.346 - 6.029) | 0.614 |
| Level of education | ||||
| Illiterate | 19 (11.9) | 17 (8.6) | Reference | |
| Under diploma/diploma | 51 (31.9) | 75 (38.1) | 0.460 (0.208 - 1.016) | 0.055 |
| University education | 90 (56.3) | 105 (53.3) | 0.464 (0.207 - 1.039) | 0.062 |
| Received antibiotics | 114 (71.3) | 187 (94.9) | 0.751 (0.677 - 0.833) | 0.098 |
| ICU admission | 6 (3.7) | 4 (2) | 0.532 (0.147 - 1.919) | 0.327 |
| Length of hospital stay, day | 8.49 ± 6.21 | 6.11 ± 11.63 | 1.121 (0.906 - 3.368) | 0.531 |
| Anxiety (HADS) | 89 (55.6) | 145 (73.6) | 2.225 (1.426 - 3.470) | < 0.001 |
| Depression (HADS) | 59 (36.9) | 69 (35.0) | 1.284 (0.799 - 2.063) | 0.301 |
Risk Factors Associated with the Development of GI Symptoms after COVID-19 a
For FD, having anxiety (OR (95% CI): 1.110 (0.522 - 2.359); P-value = 0.042) and depression (OR (95% CI): 3.753 (1.789 - 7.872); P-value < 0.001) were factors that increased the odds ratio. In contrast, being female (OR (95% CI): 0.491 (0.235 - 0.925); P-value = 0.042) and having a university education (compared to illiteracy) (OR (95% CI): 0.272 (0.099 - 0.747); P-value = 0.012) reduced the odds of developing FD (Table 4).
| Variables | FD (n = 34) | Non-FD (n = 323) | OR (95 CI) | P - Value |
|---|---|---|---|---|
| Age, y | 46.09 ± 8.45 | 49.07 ± 10.87 | 0.974 (0.942 - 1.007) | 0.120 |
| Sex | ||||
| Male | 22 (64.7) | 153 (47.4) | Reference | |
| Female | 12 (35.3) | 170 (52.6) | 0.491 (0.235 - 0.925) | 0.042 |
| Job status | ||||
| Unemployed | 2 (5.9) | 19 (5.9) | Reference | |
| Freelance employment | 12 (35.3) | 82 (25.4) | 1.390 (0.287 - 6.736) | 0.682 |
| Employed | 14 (41.2) | 96 (29.7) | 1.385 (0.291 - 6.601) | 0.683 |
| Housewife | 6 (17.6) | 112 (34.7) | 0.509 (0.096 - 2.710) | 0.853 |
| Level of education | ||||
| Illiterate | 7 (20.6) | 29 (9.0) | Reference | |
| Under diploma/diploma | 15 (44.1) | 111 (34.4) | 0.560 (0.209 - 1.500) | 0.249 |
| University education | 12 (35.3) | 183 (56.7) | 0.272 (0.099 - 0.747) | 0.012 |
| Received antibiotics | 25 (73.5) | 276 (85.4) | 2.114 (0.929 - 4.811) | 0.082 |
| ICU admission | 3 (8.8) | 7 (2.2) | 0.229 (0.056 - 0.930) | 0.064 |
| Length of hospital stay; day | 7.14 ± 10.10 | 7.07 ± 11.03 | 0.986 (0.163 - 1.967) | 0.867 |
| Anxiety (HADS) | 23 (67.6) | 211 (65.3) | 1.110 (0.522 - 2.359) | 0.042 |
| Depression (HADS) | 22 (64.7) | 106 (32.8) | 3.753 (1.789 - 7.872) | < 0.001 |
Risk Factors Associated with FD after COVID-19 a
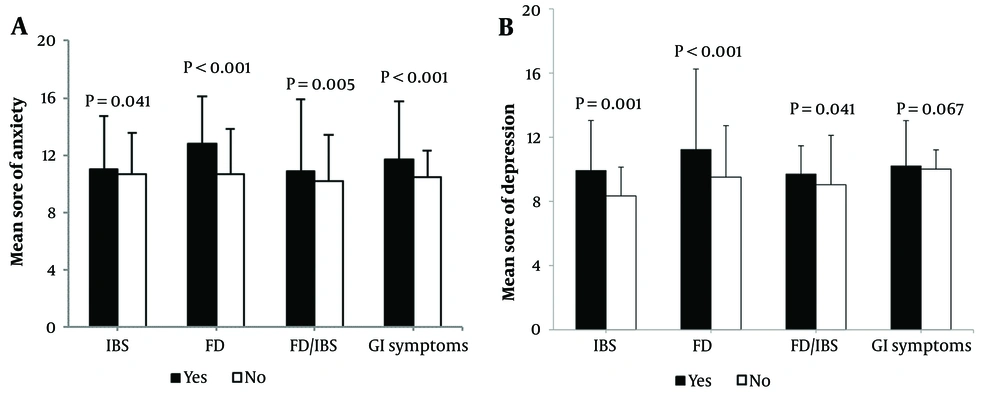
According to Figure 1, the average levels of anxiety and depression among individuals with only IBS, only FD, both IBS and FD, and GI symptoms were significantly higher than those without these conditions (P-value < 0.05). However, depression alone did not significantly differ between patients with and without GI symptoms (P-value = 0.067).
5. Discussion
The present study found that GI symptoms were more frequent than IBS and FD within one year after recovering from COVID-19. The most prevalent symptoms experienced by these patients after one year were diarrhea, nausea, vomiting, and abdominal pain. An analysis of the risk factors associated with an increased odds ratio of occurrence showed that anxiety heightened the odds ratio of developing GI symptoms post-COVID-19, and women were more likely to experience GI symptoms than men, although being a housewife was associated with a reduced odds ratio of developing GI symptoms. This suggests that job stress may contribute to tension and FGIDs in individuals, so housewives, being removed from workplace stresses, have a lower risk of developing GI symptoms after COVID-19.
These findings align with other studies indicating that, in addition to shortness of breath and fatigue, changes in bowel habits are among the common post-COVID symptoms (19, 20). Moreover, there have been reports of 3% new bowel control issues within 48 days after discharge in Italy (21), 12% persistent diarrhea and vomiting for 60 days post-COVID-19 in France (22), and 5% experiencing diarrhea and vomiting 6 months after COVID-19 in China (23), demonstrating that the occurrence of GI symptoms in the short-term or long-term after COVID-19 is evident across different races and ethnicities. Blackett et al. reported a fourfold increase in the incidence of GI symptoms at least 6 months post-COVID-19 (14) but did not identify any risk factors for new GI symptoms post-COVID-19. Similar to our study, they found no association between the length of hospital stay or ICU admission and the development of GI symptoms. Additionally, they reported, like our study, that women were more likely to develop symptoms such as abdominal pain and identified depression as a factor in the presence of at least one GI symptom (14). Previous studies have also reported that individuals with depression and anxiety are more prone to GI functional disorders (24-26). Clauw et al. suggested that COVID-19 could lead to chronic somatic diseases such as functional disorders and GI symptoms (12).
Schmulson et al. proposed that post-COVID-19 GI symptoms might be linked to persistent psychological factors like post-traumatic stress disorder, ongoing intestinal inflammation, or disruptions in the gut microbiome (27). While the role of psychiatric disorders aligns with our study's findings, further research is needed for a comprehensive understanding of the clinical, biological, and psychiatric factors involved.
On the other hand, 15.4% of patients developed IBS after recovering from COVID-19, with IBS with predominant diarrhea (IBS-D) occurring more frequently than IBS-C. Anxiety, depression, and being female have been identified as risk factors that increase the odds ratio of developing IBS, whereas freelancers and housewives were less likely to encounter this functional problem.
Blackett et al. reported an increase in the frequency of IBS-related symptoms from 16% to 41% post-COVID-19 (14). Although our study aimed to exclude patients with a history of GI functional disorders, their findings align with ours, indicating a significant increase in IBS post-COVID-19.
A study on Campylobacter-induced gastroenteritis revealed that 21% of patients developed post-infectious IBS, mostly with symptoms of diarrhea or mixed symptoms (28). Among patients with pre-existing IBS, 38% reported an increase in the frequency of abdominal pain after Campylobacter infection, paralleling our observations of IBS symptoms developing post-COVID-19 in patients with abdominal pain as a common complication.
Further research has indicated a worsening of post-COVID IBS (13). However, Zi Quek et al. have also suggested that COVID-19-related GI symptoms can mimic those of IBS. The isolation of COVID-19 patients can create stress and tension, leading to a short-term worsening of GI symptoms (29). Notably, our study found anxiety and depression to be risk factors for post-COVID IBS, possibly due to the anxiety and stress induced by COVID-19, which can have long-term effects on the GI system.
Regarding FD, although it has been less frequently studied separately, Oshima et al. have demonstrated that COVID-19 can cause or exacerbate FD, and physicians should pay close attention to patients with a history of IBS and FD symptoms (13). They also found that 4% of patients had FD-IBS, with underlying mental disorders and stress from jobs or school significantly associated with the worsening of these symptoms. Younger age and working or studying from home were also linked to symptom improvement (13); this aligns with our study’s findings, as university-educated individuals were less likely to develop FD due to a better understanding of this health issue and coping with stress. Furthermore, our study has previously shown that housekeeping is associated with a reduced odds ratio of developing IBS and GI symptoms.
It is important to highlight that this study's long-term examination (one year post-COVID) of GI symptoms across three categories— FD, IBS, and general GI symptoms—in COVID-19 patients without a prior history of GI diseases stands as one of its strengths. However, the study also encounters limitations, including the sole consideration of hospitalized patients without gathering data on inflammatory factors, blood oxygen saturation, other blood factors, and prescribed medications. Furthermore, the impact of drug abuse, smoking, and alcohol consumption, which may worsen IBS or FD, was not considered. The cross-sectional nature of this study precludes the determination of cause-and-effect relationships between the findings and the assessment of confounding factors affecting each of the FGIDs. Thus, longitudinal studies are necessary to monitor patients from the onset of infection and throughout the subsequent years.
5.1. Conclusions
In conclusion, this study reveals that one year after recovering from COVID-19, IBS-D and GI symptoms such as diarrhea, nausea, vomiting, and abdominal pain are prevalent. Furthermore, being female and experiencing anxiety and depression emerge as significant risk factors for these conditions. Additionally, employment in less stressful occupations (e.g., housekeeping) and higher educational attainment can lower the likelihood of developing these GI disorders.