1. Background
The 2019 Coronavirus disease (COVID-19) has created a health crisis of unprecedented proportions (1), which was first identified in December 2019 and declared a global pandemic in March 2020 (2). COVID-19 can lead to a mild or severe disease with a high mortality rate (3).
It seems that pregnant women and people with underlying diseases are more exposed to the complications caused by COVID-19 due to their weakened immune systems (4, 5). Among other things, respiratory problems in this group, the possibility of hospitalization, and/or the need for special care increase compared to others. Also, the probability of premature delivery and Cesarean section is higher in pregnant women with COVID-19 (6). In addition, newborns of mothers infected with COVID-19 during pregnancy are more likely to be admitted to the neonatal intensive care unit (NICU) (7-9).
During pregnancy, immunological and anatomical changes occur in the respiratory system, which leads to respiratory disorders and changes in pregnant women and affects lung function. These changes can expose pregnant women to severe respiratory infections. Therefore, paying attention to the safety of pregnant women and promoting it is vital in this pandemic (10, 11).
Control measures for this epidemic include personal hygiene, social distancing, face masks, isolation, and vaccination. Vaccination is the only realistic and practical way to protect safety against SARS-CoV-2 (12).
Vaccination is an essential part of women's care during pregnancy (13) and has been established as an important strategy to prevent maternal and infant disease and reduce the morbidity and mortality caused by the disease among pregnant women and newborns (14). Vaccination during pregnancy can directly protect both the fetus and the newborn through antibodies transferred from the mother to the fetus (9, 15, 16).
The Centers for Disease Control and Prevention (CDC) recommends that pregnant women or lactating or those planning to become pregnant be vaccinated against COVID-19 (17). Studies have demonstrated that vaccinated pregnant women have developed similar immune responses as vaccinated non-pregnant people, and higher antibody levels have been developed in pregnancy than in those observed after contracting COVID-19 (18, 19).
Information about vaccination during pregnancy is vital for pregnant women, healthcare providers, researchers, vaccine manufacturers, and community members (20, 21). In evaluating vaccine safety in pregnant women, the maternal and newborn data collection and management system should be used to provide information on the rate of pregnancy outcomes and neonatal events (22).
In an information management system (IMS), data on health are recorded, stored, retrieved, and processed to provide faster access to data and more accurate analyses for informed decision-making and performance improvement (23).
Considering that exposure to the vaccine can affect the health of the mother and the fetus, the health of the mother and the fetus/newborn/infant should be monitored, and side effects reported in the mother and the newborn/infant after the immunization should be included in the design of management systems. Therefore, the vital role of management systems in evaluating vaccination safety in pregnant women is clear (24).
2. Objectives
A vaccination IMS is required to control and prevent complications caused by vaccination. Therefore, the main goal of this study is to present the model of the IMS for COVID-19 vaccination of pregnant women as a strategy to improve the safety of pregnant women in Iran.
3. Methods
The current research was of an applied type, carried out descriptively and in three stages. In the first stage, to identify and collect the required information and explain the various components of the IMS for the vaccination of pregnant women, PubMed, Google Scholar, Springer, and Wiley Science Direct databases were used as databases. Also, a comparative study of the IMS for the vaccination of pregnant women in the United States, the United Kingdom, and Canada was conducted qualitatively. In the second stage, the initial model was designed based on the results of the first stage and according to the country's organizational structure.
Thirdly, the validation of the proposed model was examined in two steps using the Delphi technique. For this, a questionnaire was developed, the validity of which was obtained through content validity and based on the study of authentic texts and receiving experts' opinions on the research subject. The reliability of the questionnaire was also confirmed through retesting. To implement the Delphi technique, a questionnaire was given to 15 people (including ten health information management specialists and five obstetricians), all of whom were university faculty members. The acceptance criteria for the proposed model was an agreement coefficient of 80%. In the second stage of the Delphi technique, a specialized panel was formed consisting of five experts (members of the faculty of health information management), and the proposed implementations and the final model were presented in the form of a schematic diagram and were finally approved.
4. Results
The findings of this study are presented in three sections as follows:
4.1. First Part
Findings from the comparative study of the IMS for vaccinating pregnant women in selected countries.
In this part of the study, the IMS for the COVID-19 vaccination of pregnant women was examined from two points of view: Structural components (including system goals, surveillance systems, monitoring methods, responsible organization, data sources, participating centers) and Informational process (including data sets, data collection, data quality control, data exchange, data processing, reporting) in selected countries, which are shown in Tables 1 and 2.
| Components/Countries | USA | United Kingdom | Canada |
|---|---|---|---|
| System goals | Monitoring the safety of the COVID-19 vaccination in pregnant women and their infants | Monitoring the COVID-19 vaccination in pregnant women | Monitoring the safety of COVID-19 vaccination in pregnant women, evaluating the attitude towards COVID-19 vaccination in pregnant women |
| Surveillance systems | Vaccine Adverse Event Reporting System (VARES), V-safe and V-safe pregnancy registry, vaccine safety datalink (VSD), clinical immunization safety assessment (CISA) | The yellow card scheme, yellow card vaccine monitor, UK surveillance of vaccination in pregnancy (VIP), UKTIS and UKOSS surveillance of COVID-19 vaccination in pregnancy, WICH monitoring tool, the national disease registration service (NDRS) | COVID-19 vaccine registry for pregnant & lactating individuals (COVERED), the Canadian national vaccine safety network (CANVAS) |
| Monitoring method | Active and passive | Active and passive | Active and passive |
| Supervisory levels | National, state | National, regional, provincial | National, provincial, regional |
| Responsible organization | The Centers for Disease Control and Prevention (CDC) | United Kingdom Health Security Agency (UKHSA) | Public Health Agency of Canada (PHAC) |
| Data sources | Healthcare providers, clinics (obstetrics, pediatrics), imaging centers and genetics laboratories, medical records, vaccine recipients, vaccine manufacturers | Healthcare professionals or health units such as clinics and hospitals (obstetrics, pediatrics), genetics and teratology laboratories, and medical records | Healthcare providers, obstetrics clinics, medical records |
| Participating centers | US Food and Drug Administration (FDA), Center for Epidemiologic Research, Statistics Center, Maternal, Fetus, and Newborn Research Centers,Center for Infectious Diseases | Medicines and Healthcare Products Regulatory Agency (MHRA), UKTIS, Scotland Public Health, Wales Public Health and Public Health Agency in Northern Ireland, National Perinatal Epidemiology Unit (NPEU), University of Oxford and Royal College of Obstetrics | University of British Columbia (UBC), Hospital’s Women's Health Research Institute (WHRI), Vancouver Women's Health Center, Vaccine Evaluation Center, Women's and Children's Health Association, Reproductive Infectious Diseases Team, COVID-19 Safety Task Force, Provincial/Regional Vaccination Advisory Committees |
Abbreviations: UKTIS, the UK teratology information service; UKOSS, UK obstetric surveillance system; WICH, Wider impacts of COVID-19 on health.
| Processes/Countries | USA | United Kingdom | Canada |
|---|---|---|---|
| Data set | Patient demographic information, patient clinical data, information about the COVID-19 vaccine, side effects after vaccination, complications during pregnancy, newborn/infant outcomes | Patient demographic information, pregnancy information, information about the COVID-19 vaccine, side effects after vaccination, complications during pregnancy, newborn/infant outcomes | Patient demographic information, patient clinical data, information about the COVID-19 vaccine, side effects after vaccination, complications during pregnancy, newborn/infant outcomes |
| Data collection | Data collection through an electronic questionnaire form by obstetricians, pediatric infectious diseases specialists, nurses, epidemiologists, reproductive health specialists, birth defects specialists, and clinical genetics specialists | Data collection through an electronic questionnaire form byobstetricians, midwives, anesthesiologists, perinatal risk management, teratologists | Collecting data through an electronic questionnaire or sending relevant records by fax |
| Data quality control | Review of clinical data reported by multiple reviewers | Review and control of data collection forms by the steering committee | Monitoring and inspection of data by selected representatives of the principal investigator |
| Data exchange | Linking databases to each other and sending the required information | Linking databases to each other and sending the required information | Linking databases to each other and sending the required information |
| Data processing | Calculation and data processing using descriptive statistics and calculation of proportional reporting ratios (PRR) and empirical Bayesian data mining (EB) | Calculation and data processing using descriptive statistics and a pregnancy episode algorithm (PEA) | Calculation and data processing using descriptive statistics |
| Reporting | Publication of periodic reports in the form of articles and updated summaries | Publication of quarterly and annual reports | Publication of reports through website and email |
4.2. Second Part
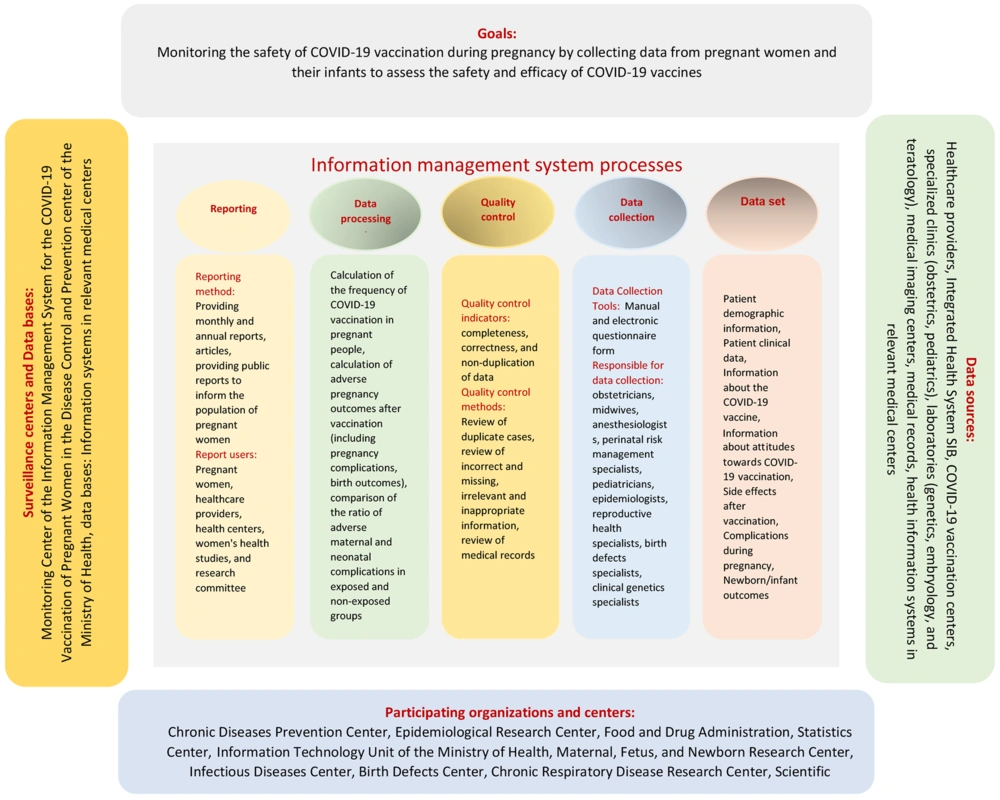
Table 3 shows the proposed model of the IMS for pregnant women's COVID-19 vaccination in Iran; the structural components (including responsible organization and databases, surveillance center, participating organizations, and data sources) and Informational process (including data sets, data collection, data quality control, data exchange, data processing, reporting).
| Variables | Values |
|---|---|
| Goals | Monitoring the safety of COVID-19 vaccination during pregnancy by collecting data from pregnant women and their infants to assess the safety and efficacy of COVID-19 vaccines |
| Structural components | |
| Responsible organization | Disease control and prevention center in the Ministry of Health |
| Participating organizations in the system | Chronic Disease Prevention Center, Epidemiological Research Center, Food and Drug Administration, Statistics Center, Maternal, Fetus, and Newborn Research Center, Information Technology Unit of the Ministry of Health, Infectious Diseases Center, Birth Defects Center, Chronic Respiratory Disease Research Center, Scientific Committee on COVID-19 |
| Data sources | Healthcare providers, integrated health system (SIB), COVID-19 vaccination centers, specialized clinics (obstetrics, pediatrics), laboratories (genetics, embryology, and teratology), medical imaging centers, medical records, health information systems in relevant medical centers |
| Databases | Information systems in relevant medical centers |
| Surveillance centers | monitoring center of the information management system for the COVID-19 vaccination of pregnant women in the disease control and prevention center of the Ministry of Health |
| Information management system processes | |
| Data sets | Patient demographic information, patient clinical data, information about the COVID-19 vaccine, information about attitudes toward COVID-19 vaccination, side effects after vaccination, complications during pregnancy, newborn/infant outcomes |
| Data collection | Based on a manual and electronic questionnaire formed by obstetricians, midwives, anesthesiologists and perinatal risk management specialists, pediatricians, epidemiologists, reproductive health specialists, birth defects specialists, clinical genetics specialists |
| Quality indicators | completeness, correctness, and non-duplication of data |
| Quality control methods | Review of duplicate cases, review of incorrect and missing, irrelevant and inappropriate information, review of medical records |
| Data exchange | Sending information summary online from information systems in urban centers to information systems in provincial and national centers |
| Types of data processing | Calculation of the frequency of COVID-19 vaccination in pregnant women, calculation of adverse pregnancy outcomes after vaccination (including pregnancy complications and birth outcomes), comparison of the ratio of adverse maternal and neonatal complications in exposed and non-exposed groups |
| Reporting and informing method | Providing monthly and annual reports, articles, providing public reports to inform the population of pregnant women |
| Report users | Pregnant women, health care providers, health centers, women's health studies, and research committee |
4.3. Third Part
Table 4 shows the findings from the validation of the proposed model of the IMS for pregnant women's COVID-19 vaccination in Iran, and Figure 1 shows the schematic form of the model for the IMS for pregnant women's COVID-19 vaccination in Iran.
| Suggested Components | Experts’ Opinions | ||
|---|---|---|---|
| The Delphi Technique | Description | ||
| Agree on Percentage | Disagree Percentage | ||
| Structural Components | |||
| Goals | 100 | - | - |
| The monitoring system ownership | 100 | - | - |
| Data sources | 87 | 13 | The integrated health system (SIB) should be used as one of the data sources to record the information |
| Participating centers | 100 | - | - |
| Databases | 100 | - | - |
| Surveillance centers | 80 | 20 | The monitoring center of the IMS for the vaccination of pregnant women against COVID-19 should be established in the disease control and prevention center of the Ministry of Health. |
| Informational processes | - | ||
| Data set | 100 | - | - |
| Data collection | 100 | - | - |
| Quality control | 100 | - | - |
| Data processing | 100 | - | - |
| Data exchange | 100 | - | - |
| Reporting | 87 | 13 | The Ministry of Health should be considered as one of the users of the reports. In the development of the software based on the proposed model, a dynamic report generator should be added to the collection. |
5. Discussion
A management and surveillance system should be used to assess and monitor the safety of COVID-19 vaccination during pregnancy (25). Considering that there is no information management system for the COVID-19 vaccination of pregnant women in Iran, therefore, in this study, the national model of the information management system for COVID-19 vaccination of pregnant women as a strategy to improve the safety of pregnant women by studying the surveillance systems of COVID-19 vaccination of pregnant women in the leading countries and the special needs of the country was prepared and presented.
The findings revealed that the IMS for the COVID-19 vaccination of pregnant women should be studied from different dimensions (26).
The proposed model of the IMS for the COVID-19 vaccination of pregnant women in Iran was analyzed from two structural and informational dimensions. Accordingly, from a structural point of view, the first point in planning an IMS is to determine ownership of the system (26). In all the countries studied the Ministry of Health takes ownership. In the proposed model of the IMS for the COVID-19 vaccination of pregnant women in Iran, the principal organization supervising the system is the disease control and prevention center in the Ministry of Health.
From a structural point of view, various surveillance systems and data sources have been formed in the studied countries according to the activity of organizations and participating centers to manage and monitor the safety of the COVID-19 vaccination in pregnant women (19, 25, 27). The proposed model of the present study presents the creation of a surveillance center in the Disease Control and Prevention center of the Ministry of Health and the creation of a database in each data center. In addition, comprehensive data sources for IMS vaccination of pregnant women in Iran are provided.
The dimension of the information process in this research includes data sets, data collection, quality control, data exchange, processing, and reporting, which are fully and clearly specified, as well as precise sub-criteria for the main components, which are comprehensively and clearly determined.
The data set is a standard tool for data collection that ensures accurate and correct health data achievement (28). The basis of any vaccine safety management and surveillance system is routine monitoring of adverse events after vaccination. When a serious adverse event is identified in a mother or newborn/infant after immunization, the information on when the vaccine was exposed during pregnancy, as well as the presence of other causes, should be collected. In addition, details of birth outcomes should be collected for further evaluation of information (24). The data set in the model of the IMS for the COVID-19 vaccination of pregnant women designed in the current study include patient demographic information and clinical data, information about the COVID-19 vaccine, information about attitudes towards COVID-19 vaccination, side effects after vaccination, complications during pregnancy, and the outcomes of the newborn/infant. Also, data collection is based on a manual and electronic questionnaire form.
Effective and efficient management of data and monitoring and control of information at different levels by management and surveillance systems leads to the production of high-quality and accurate data and the timely exchange of data and information with related organizations (29). To ensure the quality of the information in the proposed model, various information quality control methods (including review of duplicate cases, review of incorrect and missing, irrelevant and inappropriate information, and review of medical records) have been provided.
Data exchange in the studied countries is in the form of linking surveillance systems to each other and sending the required information. In the proposed model for Iran, data exchange sends a summary of information online, from information systems in urban centers to provincial and national centers.
Data analysis and interpretation are usually performed by comparing reported data with other data to detect the presence or absence of abnormalities, which may require further investigation or other actions. Considering that the data is continuously collected, monitored, and managed over time, these monitoring data can identify changes compared to the background data and analyze them (30). In the proposed model, data processing includes calculating the frequency of COVID-19 vaccination in pregnant women, calculating adverse pregnancy outcomes after vaccination (including pregnancy complications and delivery outcomes), and comparing the ratio of adverse maternal and neonatal complications in exposed groups and non-exposed groups.
Reporting information through different methods allows for comparing data at different levels for informed decision-making and appropriate actions by doctors and health stakeholders. A variety of reporting methods and report users are proposed in this study (31).
An IMS affects population health by identifying and collecting data and providing information that physicians and health stakeholders can use to improve the quality of their decisions and the effectiveness of their actions (30). An effective information management system that collects accurate, consistent, and relevant data in a timely manner can improve planning and better monitoring of health programs and service delivery and, in turn, help increase the effectiveness of appropriate interventions. On the other hand, the most appropriate data sources and data sets help to provide the required information and cost-effectiveness (32). Therefore, implementing an information management system will reduce the time spent on manual work, increase the accuracy and availability of health care data in electronic format, and as a result, it will be cost-effective and improve the effectiveness of the service (33).
Therefore, the proposed model in this study is a comprehensive and optimal tool for managing the data on the vaccination of COVID-19 in pregnant women, and the results of this model can be the basis for implementing an information management system for COVID-19 vaccination in pregnant women in the country.
5.1. Conclusions
By implementing an information management system for the vaccination of pregnant women, it is possible to provide all kinds of reports and statistics related to the health of pregnant women. Also, the possibility of various decisions by officials regarding allocating resources, monitoring the status of vaccination, and increased coordination among health service providers will be achieved. In addition, creating a rich source of data and information as an effective tool will be available to research centers and researchers to conduct clinical and epidemiological studies