1. Background
Coronaviruses are important pathogens in humans and animals. In December 2019, the severe acute respiratory syndrome (SARS-CoV-2) virus, the cause of coronavirus disease 2019 (COVID-19), spread in Wuhan, a city in Hubei province, China (1-3). SARS-CoV-2 has a lower mortality rate than its two predecessors, SARS-CoV and MERS-CoV, but it spreads faster than both. The World Health Organization (WHO) was forced to declare it a pandemic in March 2020 due to the virus’s highly contagious nature and worldwide infection (4).
Genome analyses showed that SARS-CoV-2 is a betacoronavirus, same as SARS-CoV and MERS-CoV, but in different clades (5). Angiotensin-converting enzyme 2 (ACE2) is the host cell receptor for SARS-CoV and SARS-CoV-2 entry (6). Due to the various expressions of this receptor in different organs, virus particles can cause a vast spectrum of clinical manifestations and complications (4). Atypical or organized pneumonia is the first radiological finding in COVID-19 patients. Nearly 18% of patients have normal chest X-rays and CT scans at the beginning of the disease (7). Ground-glass opacification with or without mixed consolidation, adjacent pleural thickening, intralobular septal thickening, and air bronchograms are the most frequent chest CT findings (8). Lymphopenia, increased levels of aminotransferases, increased levels of lactate dehydrogenase, and elevated inflammatory markers such as ferritin, C-reactive protein (CRP), and erythrocyte sedimentation rate (ESR) are common laboratory findings in hospitalized patients (9).
In April 2020, an analysis of 19 studies by Rodriguez-Morales et al. showed that rapid progression of fever, cough, and dyspnea are common symptoms of COVID-19, and rapid progression to acute respiratory distress syndrome (ARDS) is among the more specific manifestations of the disease. In terms of imaging, bilateral pneumonia and ground-glass opacification were the most common findings (10). Another study conducted in November 2020 by Allameh et al. reported fever, dry cough, and dyspnea as the most common signs, similar to previous studies. However, the proportion of these symptoms in the patients of this study was higher than in other studies. For example, 93.5% of patients had a fever, which was less frequently reported in previous studies. Additionally, laboratory findings showed increased levels of LDH or CRP in more than 90% of patients, but lymphopenia was reported in only 42.9% of patients (11).
Finally, although it seems the symptoms of patients with COVID-19 have been similar since the beginning, different studies have shown varying manifestations over time. These changes in symptoms could be the result of genomic changes in the virus, temperature changes, or racial differences in infected people at different times. As far as we know, few reliable studies with access to a large sample size of patients have compared the symptoms during different peaks of this disease in Iran. Evaluating these changes can help public health decision-makers and clinicians to manage the disease in society and individuals more effectively.
2. Methods
We conducted this cross-sectional study on hospitalized COVID-19 cases at Imam Reza Hospital, Mashhad, Iran, in 2021. This study was approved by the Mashhad University of Medical Sciences Ethics Committee (code of ethics: IR.MUMS.REC.1399.572). We included all patients older than 18 years with confirmed COVID-19 infection based on clinical features, CT scan findings, and positive COVID PCR test from February 20, 2020, until December 20, 2020. We excluded pregnant and pediatric cases. Based on the number of hospitalized patients meeting the study criteria at our center and the prevalence of this disease in the community, patients were divided into three separate groups: The first peak from February 2020 to June 2020, the second peak from June 2020 to September 2020, and the third peak from September 2020 to December 2020 (12).
We used a pre-prepared checklist for gathering data on our variables. Our variables were divided into three categories: (1) Demographic information, including age, gender, and comorbidities (e.g., diabetes, hypertension, cardiovascular, pulmonary, hepatic, renal disease, malignancies, and HIV); (2) physical examination and clinical manifestations, including fever, cough, fatigue, headache, hemoptysis, diarrhea, vomiting, nausea, dyspnea, hypertension, tachypnea (respiratory rate > 24), and abdominal pain. These signs and symptoms were assessed using first admission data.; (3) disease severity was based on O2 saturation at the time of admission, complete blood count (CBC), ESR and CRP values, CT scan score (measured by the amount of lung involvement from 0 to 5 in each of the 5 lobes of the lung and then summed up to give a score of 0 to 25, based on the study by Pan et al. (13), ICU admission, and outcome. The obtained data were analyzed using SPSS software version 25. The comparison of qualitative data between the three peaks was done using the chi-square test, and quantitative data were analyzed with the ANOVA test. The estimated number of patients in each of the three peaks was 150.
3. Results
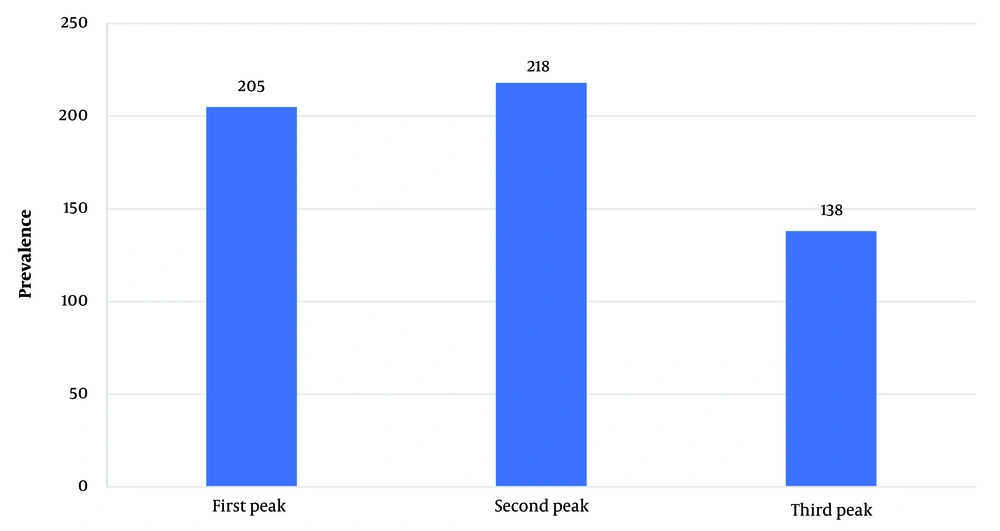
In total, we included 561 patients in this study with a mean age of 58.9 ± 16.8 years. Our study population consisted of 336 males (59.9%) and 225 females. The second peak had the most admitted patients (218 patients, 38.6%), while the lowest number of patients was in the third peak (138 patients, 24.6%) (Figure 1).
Hypertension and diabetes were the most common comorbidities, with 293 (52.2%) and 226 (40.3%) cases respectively. Dyspnea was the most frequent symptom, present in 462 (75.4%) patients at the time of admission, followed by fever in 423 (75.4%) patients. The average time elapsed since the onset of symptoms was 7.12 ± 2.37 days. Among the patients included in the study, the average CT score was 12.26 ± 4.95. As seen in Table 1, ground glass opacity was the most common finding on CT scans, present in 537 patients (96.6%).
| Data | No. (%) |
|---|---|
| Gender | |
| Male | 336 (59.9) |
| Female | 225 (40.1) |
| Cigarette smoking | 144 (25.7) |
| Drug addiction | 56 (10) |
| Alcohol consumption | 3 (0.5) |
| Underlying disease | |
| Diabetes | 226 (40.3) |
| Hypertension | 293 (52.2) |
| Asthma | 13 (2.3) |
| Ischemic heart disease | 171 (30.4) |
| Chronic kidney disease | 42 (7.5) |
| Transplantation | 13 (2.3) a |
| Malignancy | 26 (4.6) |
| COPD | 67 (11.9) |
| CVD | 15 (2.7) |
| Other conditions b | 41 (7.3) |
| Drugs | |
| Anti hypertension (except ACE, ARB) | 311 (55.4) |
| ACE I or ARB | 240 (42.8) |
| Anti diabetes | 220 (39.2) |
| Chemotherapy | 12 (2.1) |
| Corticostroides | 18 (3.2) |
| Aspirine | 15 (2.7) |
| Nonsteroidal immunosuppresives | 12 (2.1) |
| Clinical features | |
| Fever | 423 (75.4) |
| Cough | 333 (59.4) |
| Dyspnea | 462 (82.4) |
| Phlegm | 53 (9.4) |
| Chest discomfort | 302 (53.8) |
| Nausea | 118 (21) |
| Vomiting | 109 (19.4) |
| Diarrhea | 50 (8.9) |
| Rhinorrhea | 4 (0.7) |
| Abdominal pain | 34 (6.1) |
| Conjunctivitis | 3 (0.5) |
| Headache | 300 (53.5) |
| Myalgia | 219 (39) |
| Arthralgia | 73 (13) |
| Weakness | 386 (68) |
| Fatigue | 156 (27.8) |
| Sore throat | 31 (5.5) |
| Shaking | 46 (8.2) |
| Confusion | 90 (16) |
| Anosmia | 21 (3.7) |
| Taste disorder | 4 (0.7) |
| CT scan findings | |
| Ground glass | 537 (96.6) |
| Consalidation | 394 (70.9) |
| Crazy paving | 120 (21.6) |
Demographic, Comorbidities Details, Patients’ Signs and Symptoms, and Imaging Findings
Demographic details, comorbidities, patients’ signs and symptoms, and imaging findings are shown in Table 1. Table 2 shows the details of patients' vital signs and laboratory findings.
| Variables | Mean ± Standard Deviation | Highest | Lowest |
|---|---|---|---|
| Heart rate | 109.66 ± 17.49 | 150 | 29 |
| Respiratory rate | 30.02 ± 6.54 | 100 | 15 |
| Systolic blood pressure | 128.34 ± 20.44 | 200 | 70 |
| Diastolic blood pressure | 82.04 ± 11.39 | 130 | 50 |
| SPO2 | 82.72 ± 7.66 | 98 | 60 |
| Hemoglobin (gr/dL) | 12.96 ± 2.75 | 45.8 | 4.5 |
| WBC (109/L) | 9.93 ± 11.65 | 0.3 | 240 |
| Neutrophile (%) | 79.58 ± 11.33 | 99 | 8 |
| Lymphocytes (%) | 14.66 ± 9.42 | 76 | 1 |
| Platelets (103/µL) | 213.33 ± 104.09 | 917 | 2.3 |
| ESR | 52.75 ± 30.76 | 196 | 1 |
| CRP | 107.9 ± 84.4 | 554 | 0.1 |
Patients’ Vital Signs and Laboratory Findings Details
Smoking (P < 0.001), diabetes (P = 0.003), hypertension (P < 0.001), asthma (P = 0.025), ischemic heart disease (P < 0.001), chronic kidney disease (P = 0.003), and transplantation (P = 0.007) showed significant differences between the first, second, and third peaks. Gender, opioid usage, alcohol consumption, malignancy, chronic obstructive pulmonary disease (COPD) and cardiovascular disease (CVD) did not show significant differences across different peaks (Table 3).
| Variables | P-Value | First Peak | Second Peak | Third Peak |
|---|---|---|---|---|
| Gender | 0.995 b | |||
| Male | 123 (60) | 130 (59.6) | 83 (60.1) | |
| Female | 82 (40) | 88 (40.4) | 55 (39.9) | |
| Cigarette smoking | < 0.001 b | 30 (14.6) | 68 (31.2) | 46 (33.3) |
| Drug addiction | 0.283 b | 16 (7.8) | 22 (10.1) | 18 (13) |
| Alcohol consumption | 0.939 b | 1 (0.5) | 1 (0.5) | 1 (0.7) |
| Underlying disease | ||||
| Diabetes | 0.003 b | 65 (31.7) | 93 (42.7) | 68 (49.3) |
| Hypertension | < 0.001 b | 65 (31.7) | 140 (64.2) | 88 (63.8) |
| Asthma | 0.025 b | 9 (4.4) | 4 (1.8) | 0 (0) |
| Ischemic heart disease | < 0.001 b | 39 (19) | 90 (41.3) | 42 (30.4) |
| Chronic kidney disease | 0.003 b | 5 (2.4) | 23 (10.6) | 14 (10.1) |
| Transplantation | 0.007 b | 3 (1.5) | 2 (0.9) | 8 (5.8) |
| Malignancy | 0.442 b | 12 (5.9) | 10 (4.6) | 4 (2.9) |
| COPD | 0.111 b | 17 (8.3) | 29 (13.3) | 21 (15.2) |
| CVD | 0.392 b | 3 (1.5) | 7 (3.2) | 5 (3.6) |
| Age | < 0.001 c | 56.26 ± 16.18 | 62.56 ± 17.01 | 57.01 ± 166.44 |
Comparison of Patients’ Demographic and Underlying Diseases in First, Second, and Third Peaks a
The mean age during the first, second, and third peaks was 56.26 ± 16.18, 62.56 ± 17.01, and 57.01 ± 16.44 years, respectively. An ANOVA test showed significant differences in these ages (P < 0.001) (Table 4). However, post hoc analysis using the LSD test showed significant differences between the mean ages at the first and second peaks (P < 0.001) and between the second and third peaks (P = 0.002), but no significant difference between the first and third peaks (P = 0.685) (Table 4).
| Variables | First Peak | Second Peak | Third Peak | P- Value b Total | P-Value c 1 & 2 Peak | P- Value c 1 & 3 Peak | P- Value c Peak 2 & 3 |
|---|---|---|---|---|---|---|---|
| Fever | 140 (68.3) | 153 (70.2) | 130 (94.2) | 0.001 < | 0.675 | < 0.001 | < 0.001 |
| Cough | 124 (60.5) | 125 (57.3) | 84 (60.9) | 0.738 | 0.533 | > 0.999 | 0.581 |
| Dyspnea | 166 (81) | 185 (80.3) | 121 (87.7) | 0.164 | 0.902 | 0.104 | 0.081 |
| Phlegm | 34 (16.6) | 10 (4.6) | 9 (6.5) | 0.001 < | < 0.001 | 0.007 | 0.472 |
| Chest discomfort | 61 (19.8) | 156 (71.6) | 85 (61.6) | < 0.001 | < 0.001 | < 0.001 | 0.063 |
| Nausea | 34 (16.6) | 60 (27.5) | 24 (17.4) | 0.011 | 0.007 | 0.884 | 0.030 |
| Vomiting | 29 (14.1) | 53 (24.3) | 27 (19.6) | 0.031 | 0.010 | 0.233 | 0.362 |
| Diarrhea | 19 (9.3) | 25 (11.5) | 6 (4.3) | 0.070 | 0.525 | 0.094 | 0.021 |
| Rhinorrhea | 3 (1.5) | 1 (0.5) | 0 (0) | 0.244 | 0.358 | 0.277 | > 0.999 |
| Abdominal pain | 8 (3.9) | 17 (7.8) | 9 (6.5) | 0.236 | 0.102 | 0.315 | 0.835 |
| Headache | 72 (35.1) | 136 (62.4) | 92 (66.7) | 0.001 < | < 0.001 | < 0.001 | 0.430 |
| Myalgia | 86 (42) | 92 (42.2) | 41 (29.7) | 0.035 | > 0.999 | 0.023 | 0.019 |
| Arthralgia | 24 (11.7) | 31 (14.2) | 18 (13) | 0.745 | 0.472 | 0.739 | 0.875 |
| Weakness | 117 (57.1) | 170 (78) | 99 (71.7) | 0.001 < | < 0.001 | 0.006 | 0.206 |
| Fatigue | 56 (27.3) | 60 (27.5) | 40 (29) | 0.938 | > 0.999 | 0.806 | 0.809 |
| Sore throat | 22 (10.7) | 3 (1.4) | 6 (4.3) | 0.001 > | < 0.001 | 0.043 | 0.095 |
| Chills | 35 (17.1) | 3 (1.4) | 8 (5.8) | < 0.001 | < 0.001 | 0.002 | 0.026 |
| Confusion | 12 (5.9) | 53 (24.3) | 25 (18.1) | < 0.001 | < 0.001 | 0.001 | 0.190 |
| Anosmia | 2 (1) | 15 (6.8) | 4 (2.9) | 0.005 | 0.002 | 0.225 | 0.146 |
| Ageusia | 2 (1) | 1 (0.5) | 1 (0.7) | 0.819 | 0.613 | > 0.999 | > 0.999 |
| ICU admission | 28 (13.9) | 40 (18.5) | 78 (56.5) | < 0.001 | 0.233 | < 0.001 | < 0.001 |
| Mortality | 30 (14.7) | 52 (24.8) | 54 (39.7) | < 0.001 | 0.013 | < 0.001 | 0.004 |
| Mean age | 56.26 ± 16.18 d | 62.56 ± 17.01 d | 57.01 ± 16.44 d | < 0.001 e | < 0.001 f | 0.685 f | 0.002 f |
| Ct score | 10.76 ± 3.98 | 11.86 ± 4.8 | 5.3 ± 15.17 | 0.001 < g | 0.029 h | < 0.001 h | < 0.001 h |
Comparison of Patients’ Sign and Symptoms in First, Second, and Third Peaks a
Patients' signs and symptoms, including fever (P < 0.001), phlegm (P < 0.001), chest discomfort (P < 0.001), nausea (P = 0.011), vomiting (P = 0.031), headache (P < 0.001), myalgia (P = 0.035), weakness (P < 0.001), sore throat (P < 0.001), shaking (P < 0.001), confusion (P < 0.001), and anosmia (P = 0.005), showed significant differences across different peaks (Table 4).
Table 4 also compares the location of the hospitalization ward and patients' outcomes at different peaks. Both variables had significant differences between different peaks (P < 0.001).
The comparison of patients' vital signs at different peaks showed that pulse rate, respiratory rate, diastolic blood pressure, and SPO2 had significant differences. Post hoc analysis conducted for pairwise comparisons of these variables revealed significant differences in blood O2 saturation, diastolic blood pressure, respiratory rate, and heart rate between the first and second peaks; blood O2 saturation, respiratory rate, and heart rate between the first and third peaks; and blood O2 saturation between the second and third peaks.
Fever (P < 0.001), phlegm (P < 0.001), chest discomfort (P < 0.001), nausea (P = 0.011), vomiting (P = 0.031), headache (P < 0.001), myalgia (P = 0.035), weakness (P < 0.001), sore throat (P < 0.001), shaking (P < 0.001), confusion (P < 0.001), and anosmia (P = 0.005) were patients' signs and symptoms that had significant differences across different peaks.
The average CT scan scores in the first, second, and third peaks were 10.76 ± 3.98, 11.86 ± 4.8, and 5.3 ± 15.17, respectively. Welch's analysis test showed that these scores had significant differences (P < 0.001).
Post hoc analysis using the Games-Howell test for CT scores showed significant differences between the first and second peaks (P = 0.029), the first and third peaks (P < 0.001), and the second and third peaks (P < 0.001). CT scan findings indicated that the prevalence of consolidation and crazy paving showed significant differences across different peaks. Table 4 compares the location of the hospitalization ward and patients' outcomes at different peaks, showing significant differences between these variables (P < 0.001). Table 4 also compares patients' signs and symptoms across different peaks.
A comparison of laboratory findings showed that patients' white blood cell (WBC) count, neutrophil percentage, lymphocyte percentage, and CRP levels had significant differences at different peaks. Post-hoc analysis revealed significant differences in neutrophil (P < 0.001) and lymphocyte (P < 0.001) percentages between the first and second peaks; WBC count (P = 0.006), neutrophil (P < 0.001) and lymphocyte (P < 0.001) percentages, and CRP (P < 0.001) between the first and third peaks; and neutrophil percentage (P = 0.035) and CRP (P = 0.039) between the second and third peaks.
4. Discussion
After the spread of COVID-19 around the world, new variants of SARS-CoV-2 emerged, sometimes presenting different symptoms and disease severity compared to the original virus (14). This study was conducted to compare the clinical symptoms, laboratory findings, CT scans, and outcomes of patients with COVID-19 during the first three peaks of the disease in Iran, which occurred in the spring, summer, and autumn of 2020.
Our study results showed that the second peak in summer 2020 had the highest mortality rate, while the first peak during spring had the lowest mortality rate. In all three peaks, approximately 60% of hospitalized patients were male and 40% were female, with no significant difference between genders across all peaks. In terms of age, patients in the second peak were significantly older than those in the other peaks.
Cigarette smoking showed significant differences across the peaks, while alcohol consumption and opioid usage did not vary significantly in all three peaks. Except for COPD and CVD, all other comorbidities showed significant differences across the three peaks. Overall, these results indicate that patients with underlying diseases were less affected during the first peak. This may be due to the stricter adherence to health protocols by these patients during the first peak. However, in the second and third peaks, after the widespread transmission of the virus at the community level, these individuals were eventually infected.
An inquiry into patients' vital signs at admission showed a deterioration in their clinical condition over time. The deterioration of patients' vital signs in the second and third peaks is likely directly related to their comorbidities. By studying 7,000 patients, Brojakowska et al. showed that obesity, hypertension, diabetes, and coronary artery disease were the most frequent comorbidities (15). In another article published by Osibogun et al., with the same gender ratio as ours, hypertension and diabetes were more frequent than other comorbidities. Contrary to Osibogun et al.'s article, mortality was higher in our study, which can be attributed to the examination of outpatients in their study (16).
A comparison of patients' signs and symptoms showed that dyspnea was the most common symptom in the first and second peaks, but in the third peak, fever was the most common symptom and dyspnea was the second. In the third peak, nearly 95% of patients had a fever, while this rate was nearly 70% in the first and second peaks. During our first peak, Guan et al. showed that fever, non-productive coughs, and fatigue were the most frequent symptoms, respectively (17). In another study conducted by Popov et al. at the same time as our first peak, they examined the clinical features of COVID-19 patients. Similar to our study, they found that fever, cough, and headache were the most frequent symptoms, except for dyspnea (18). In another study, Valladares-Garrido et al. showed that patient symptoms such as respiratory and gastrointestinal issues, dyspnea, and ageusia were higher in the second COVID-19 wave than in the first one (19).
Among the laboratory findings, the average number of leukocytes and neutrophil percentage were highest in the third peak and lowest in the first peak. Conversely, lymphopenia was highest in the third peak and lowest in the first peak. The average CRP also increased from the first to third peaks, but there was no significant change in average ESR. The highest CT score was in the third peak and the lowest was in the first. Ground-glass opacity was the most common CT scan pathological finding. Guan et al. found pathological findings in 71% of their participants (17).
In terms of hospitalization location, ICU admissions increased from 14% in the first peak to 18% in the second peak and over 56% in the third peak. Regarding patient outcomes, less than 15% of hospitalized patients died in the first peak, over 24% died in the second peak, and nearly 40% of inpatients died in the third peak. Our study showed different mortalities between the first and third surges and the second and third surges. In contrast, Hadadi et al.'s study did not show significant differences in disease severity, ICU admission, and mortality between peaks (12).
However, a study by Jarrett et al. found that clinical outcomes such as the need for renal replacement therapy, intubation, and inpatient mortality remained unchanged between the first and second peaks (20). In another study conducted by Gray et al. in England, there was a decrease in mortality rates during both the first and second peaks (21). Similar to our study, Kumar et al. found that mortality rates were higher during the second peak than the first; however, this increase occurred mainly among younger people without underlying diseases (22).
Based on these findings, it appears that disease severity among hospitalized individuals was higher during the third peak than in previous peaks and higher during the second peak than in the first. Various factors could contribute to this situation. For example, more potent variants of the virus could lead to increased patient deaths. Another possible explanation is that due to a sharp increase in patient numbers during the second and third peaks, only critically ill patients were admitted, resulting in higher mortality rates among those hospitalized during these periods.
4.1. Limitations
The cross-sectional nature of the study and the lack of patient follow-up were the most significant limitations. However, the large sample size and the examination of patients' symptoms and findings across different peaks, which can be considered an innovation, were the strengths of our study. We suggest that future researchers examine symptoms, laboratory findings, and patient imaging during subsequent peaks of COVID-19 spread.
4.2. Conclusions
Patients’ gender showed no differences across the three peaks, although the mean age of patients was higher in the second peak than in the other two. Comorbidities were more prevalent in the second and third peaks compared to the first one. The reason for the higher mean age of patients in the second peak remains unknown. Patients’ vital signs were more unstable in the second and third peaks than in the first one. Since the patients hospitalized in the second and third peaks had more underlying diseases, the instability of their vital signs was expected. The highest patient mortality was observed in the third peak, while the lowest was in the first one.