1. Background
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was initially detected in Wuhan, China, in late 2019 and quickly disseminated worldwide. The predominant manifestation of the virus was upper respiratory tract infections. While most individuals experience mild to moderate symptoms, approximately 10% to 15% develop severe symptoms, necessitating intensive care unit (ICU) admission and mechanical ventilation (1).
While COVID-19 primarily affects the respiratory system, it also significantly impacts various organs, particularly in individuals with severe pneumonia and acute respiratory distress syndrome (ARDS). The organs affected are the gastrointestinal tract, liver, kidneys, cardiovascular, central nervous, and musculoskeletal systems (2). However, research on the influence of COVID-19 on endocrine organs remains limited. Adrenal insufficiency following COVID-19 infection may occur through various potential pathways (3).
As a result, endocrine organs are expected to be affected, particularly in individuals with severe disease cases. Additionally, the overproduction of pro-inflammatory cytokines (such as tumor necrosis factor, IL1, IL6) leads to heightened vascular permeability and contributes to the dysfunction of multiple organs (4-6).
Consequently, excessive production of pro-inflammatory cytokines can impact the hypothalamus-pituitary-adrenal (HPA) axis, primarily due to the absence of protection from the blood-brain barrier for the pituitary gland (7-10). Similar to influenza viruses, SARS-CoV-2 triggers the expression of a viral amino acid closely resembling host adrenocorticotropic hormone (ACTH) molecules. Antibodies generated against the virus attach to the viral amino acid and recognize and bind to the host's ACTH due to their molecular resemblance. This binding restricts the effectiveness of the host's ACTH in stimulating corticosteroid secretion as a response to stress (11).
One of the previous strains of the Coronavirus was responsible for the 2002 SARS outbreak. Examination of individuals affected by SARS revealed the presence of necrotic cells in the adrenal cortex. This indicates that the SARS-CoV virus can directly damage the adrenal tissue (12, 13). Numerous case reports and published studies have highlighted adrenal insufficiency as a common occurrence in COVID-19 patients (14-16). Conversely, dexamethasone and hydrocortisone administration can reduce hospitalization duration and mortality rates among COVID-19 patients (17).
Recent studies have provided limited evidence in this regard, with only a few case reports addressing adrenal insufficiency. However, there is a lack of comprehensive investigations directly examining adrenal insufficiency using validated laboratory tests. Previous research primarily focused on measuring baseline serum cortisol levels, with limited exploration of the cosyntropin test. In response to this gap, our study was designed in the spring of 2021 to assess baseline cortisol levels and evaluate the responses to cosyntropin tests in COVID-19 patients at Vali-Asr Hospital in Birjand, Iran.
2. Methods
2.1. Inclusion Criteria
We included COVID-19 patients hospitalized in Valiasr Hospital of Birjand during the spring of 2021. These patients had a confirmed diagnosis of COVID-19 using PCR testing of nasopharyngeal samples. To meet the inclusion criteria, patients were required to have an oxygen saturation (SpO2) level between 88% and 93% and exhibit less than 50% lung involvement based on HRCT reports, indicating a moderate level of involvement. We specifically excluded outpatients and individuals with severe or critical manifestations to minimize potential biases associated with disease severity and multiorgan involvement, which could impact cortisol levels.
2.2. Exclusion Criteria
We excluded individuals who exhibited the following criteria from our study:
• Those who expressed unwillingness to participate in the research.
• Individuals with a documented history of chronic corticosteroid use within the past six months.
• Patients with a medical history of adrenal insufficiency who had used corticosteroids last week.
• Patients who have been re-hospitalized due to COVID-19 because it was possible to receive corticosteroids in the previous hospitalization.
2.3. Sample Size
Since, at the time of conducting the research, we did not find a similar study to determine the sample size and studies were limited to only a few case reports, we included all hospitalized patients with a confirmed diagnosis of COVID-19 who met our specific inclusion and exclusion criteria over three months. Since most of these patients had previously received corticosteroid treatment as part of their outpatient care, our study ultimately enrolled 60 patients.
To proceed with the clinical research, researchers visited the trial site every day after receiving research certificates and the approval of the university's Ethical Committee. They examined the patients based on the inclusion and exclusion criteria. If the patients met all the criteria, they explained the program process to them, and they were included in the program after signing a written letter of consent. Demographic data such as age, gender, and coexisting conditions (such as diabetes and high blood pressure history) were documented in predesigned questionnaire forms. The initial vital signs recorded during hospitalization encompassed systolic and diastolic blood pressures and the initial sodium and potassium levels extracted from the medical records (18). Then, the patients were assigned a number from 1 to 120 to enter the program, and only those with an even number were accepted. Each participant had five milliliters of peripheral blood drawn at 8 a.m. The blood was stored in tubes containing the anticoagulant EDTA. Then, the site was examined for signs of bleeding or hematoma. The collection of slithery serum was avoided. The samples were placed in a cold environment or on Ice. Then, 250 micrograms of cosyntropin were injected intramuscularly, and another round of blood samples was collected from peripheral areas after 60 minutes. Both samples were moved to Imam Reza Hospital's central laboratory as planned.
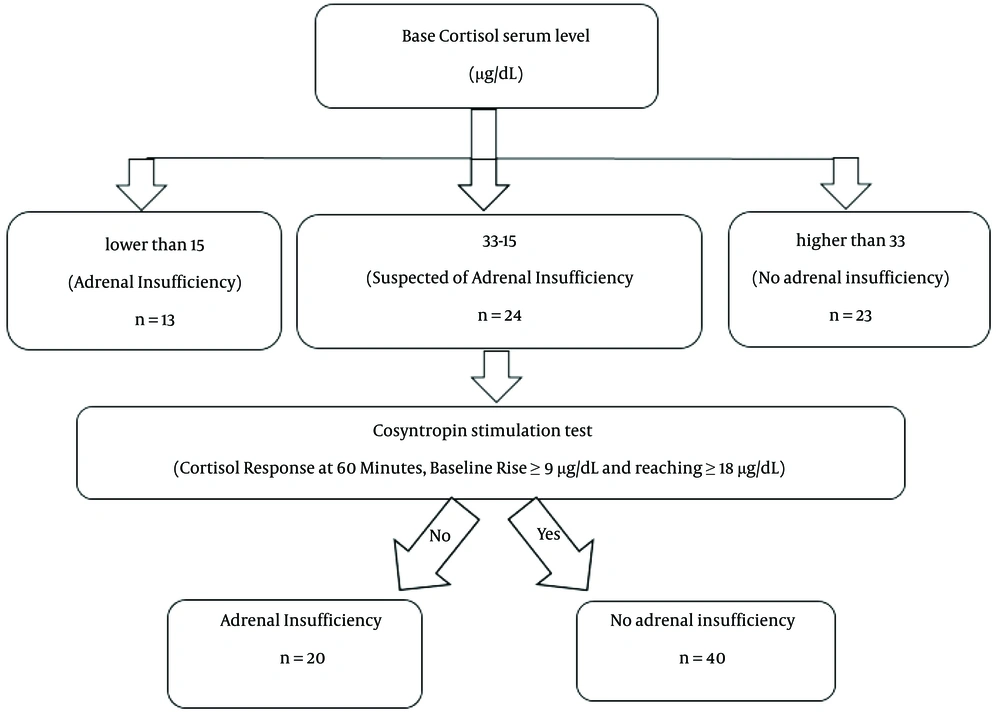
Plasma was immediately removed from each sample and stored in a freezer. Cortisol was measured using the Italian company's Saluggia kits through chemiluminescence (19, 20), and the results were recorded. In critical patients, base cortisol serum levels lower than 15 µg /dL indicate adrenal insufficiency. People with base cortisol serum levels between 15 and 33 µg /dL are suspected of adrenal insufficiency and require cosyntropin stimulation tests. Base Cortisol levels above 33 µg /dL are signs of normal adrenal function. After the cosyntropin stimulation test, a rise from the baseline of at least 9 μg/dL of cortisol, reaching at least 18 μg/dL at 60 minutes post-stimulation, effectively rules out primary adrenal insufficiency and suggests minimal adrenal suppression. A blunted or absent response indicates some level of adrenal insufficiency (21, 22). Figure 1 provides a visual representation of the sequential diagnostic process.
Following our findings, patients identified with adrenal insufficiency received treatment under the care of an endocrinologist. Furthermore, discharge status and any occurrences of in-hospital mortality were systematically assessed and documented using information from their medical records.
2.4. Data Analysis Methods
2.4.1. Statistical Analysis
All statistical tests were performed using SPSS version 22.0 for Windows (SPSS Inc., Chicago, IL, USA). The normal distribution of data was assessed using the Kolmogorov-Smirnov test. Continuous variables were expressed as mean ± standard deviation (S.D.) or median [Q1 - Q3], and categorical variables were defined as frequency (%). Differences in categorical variables were compared between groups using the chi-square test and Fisher's Exact test as needed. Unpaired samples were compared using Student's t-test or Mann-Whitney U test as required. A significance level of 0.05 was considered for all statistical analyses.
3. Results
According to the findings, a significant proportion of the patients (20 %) fell within the age range of 61 - 70 years, while a small percentage (5 %) were younger than 20. The mean age of the participants was calculated to be 63.1 ± 20.93 years.
The gender distribution of patients showed that 53.3% (n = 32) were male, and 46.7% (n = 28) were female. The median base cortisol serum levels for men were determined to be 28.075 µg/dL, while for women, the median was 21.820 µg/dL. However, no significant difference was observed in cortisol levels between men and women (P-value = 0.240).
The connection between the median baseline serum cortisol level and patients' age was investigated, but the analysis did not find any statistically significant correlation between these two factors (P-value = 0.05).
The median baseline and 60-minute serum cortisol levels were determined as 23.87 and 41.89, respectively. Analyzing these mean serum cortisol levels unveiled a statistically significant outcome differentiation (Value < 0.001).
The results showed that 21.7% (13 individuals) had adrenal insufficiency, 40% (24 individuals) were suspected of adrenal insufficiency and underwent cosyntropin stimulation tests, and 38.3% (23 individuals) did not have adrenal insufficiency. Finally, after the cosyntropin stimulation test, Adrenal insufficiency was observed in 33.3% of our patients with moderate COVID-19.
We compared the incidence of adrenal insufficiency among both sexes in the patient population. However, the findings showed no statistically significant relationship (P-value = 0.46) between gender and the incidence of adrenal insufficiency.
An evaluation of age differences among patients with adrenal insufficiency was carried out. However, the analysis identified no significant distinction between age groups (P-value = 0.84).
No statistically significant correlation was observed between underlying conditions such as diabetes and high blood pressure and the occurrence of adrenal insufficiency in patients (P-value = 0.995 and P-value = 0.685, respectively).
Upon analyzing vital signs, patients with adrenal insufficiency exhibited lower mean systolic and diastolic blood pressures, highlighting a substantial correlation when comparing these two groups (P-value < 0.001 and P-value = 0.001, respectively).
Regarding comparing sodium and potassium levels between the two groups, patients with adrenal insufficiency demonstrated lower mean serum sodium levels. In patients with normal adrenal gland function, 22.5% had hyponatremia, and 22.5% had hypernatremia. Conversely, 75% of patients with adrenal insufficiency presented hyponatremia, and none showed hypernatremia (P-value < 0.001). In contrast, the two groups had no noteworthy difference in potassium levels (P-value = 0.587).
In analyzing the data related to in-hospital mortality, it was observed that 15% of our patients with adrenal insufficiency died. The figure was 5% among coronary patients with regular adrenal function. However, no significant difference was noted (P-value = 0.322).
Table 1 provides a detailed comparison of demographic and clinical characteristics between all patients and those with adrenal Insufficiency. It covers variables such as gender, age categories, medical conditions, blood pressure, and electrolyte levels, along with in-hospital mortality. Statistical significance is evaluated through chi-square (Fisher exact) and Mann-Whitney tests, providing key insights into variable distribution among the studied groups.
| Variables | All | Adrenal Insufficiency | P-Value | |
|---|---|---|---|---|
| Yes, (N = 20) | No, (N = 40) | |||
| Genderb | 0.714 | |||
| Male | 32 (53.3) | 10 (50) | 22 (55) | |
| Female | 28 (46.7) | 10 (50) | 18 (45) | |
| Ageb | 0.279 | |||
| 20 - 50 y | 18 (30) | 8 (40) | 10 (25) | |
| 50 - 80 y | 26 (43.3) | 9 (45) | 17 (42.5) | |
| > 80 y | 16 (26.7) | 3 (15) | 13 (32.5) | |
| Age (y)c | 63.10 ± 20.93, 64.5 [43.75 - 80] | 55.40 ± 19.96, 59 [35.25 - 69.5] | 66.95 ± 20.57, 74.5 [49 - 81.75] | 0.035* |
| Diabetesb | 13 (21.7) | 4 (20) | 9 (22.5) | 0.995 |
| Hypertensionb | 17 (28.3) | 5 (25) | 12 (30) | 0.685 |
| Systolic blood pressure (mmHg)c | 113.08 ± 24.48, 110 [95 - 130] | 97.5 ± 20.93, 95 [85 - 100] | 120.88 ± 22.50, 120 [100 - 133.75] | < 0.001* |
| Diastolic blood pressure(mmHg) c | 71.5 ± 15.74, 72.5 [55 - 85] | 62.0 ± 15.08, 57.5 [55 - 68.75] | 76.25 ± 13.95, 77.5 [70 - 85] | 0.001* |
| Sodium (mEq/L) c | 137.65 ± 8.60, 135.5 [131 - 143] | 130.85 ± 4.25, 131 [128.25 - 134.75] | 141.05 ± 8.22, 140 [135 - 145] | < 0.001* |
| Potassium (mEq/L) c | 4.17 ± 0.69, 4.15 [3.8 - 4.5] | 4.16 ± 0.75, 4.25 [3.53 - 4.5] | 4.18 ± 0.67, 4 [3.83 - 4.4] | 0.587 |
| Sodiumb | < 0.001* | |||
| Hypo | 24 (40) | 15 (75) | 9 (22.5) | |
| Normal | 27 (45) | 5(25) | 22 (55) | |
| Hyper | 9 (15) | 0 | 9 (22.5) | |
| Potassiumb | 0.516 | |||
| Hypo | 8 (13.3) | 4 (20) | 4 (10) | |
| Normal | 46 (76.) | 14 (70) | 32 (80) | |
| Hyper | 6 (10) | 2 (10) | 4 (10) | |
| In hospital deathb | 5 (8.3) | 3 (15) | 2 (5) | 0.322 |
Comparison of Demographic and Clinical Variables Between Patients a
4. Discussion
This study investigated the possibility of adrenal insufficiency in patients with COVID-19. To achieve this goal, we measured baseline cortisol levels and response to cosyntropin injection. Adrenal insufficiency was detected in 33.3% of our patients with moderate involvement of COVID-19.
Sixty patients participated in this study: 32 men (%53.3) and 28 women (%46.7). The mean age of the participants was 63.1 ± 20.93. Our results indicated that the median base cortisol serum levels for men were 28.075 µg/dl, while for women, the median was 21.820 µg/dl. No significant difference in base cortisol serum levels between men and women was observed (P-value = 0.240). No significant statistical correlation was observed (P-value = 0.05) when examining the association between the median baseline serum cortisol level and the age of the patients. No significant association was observed between the sex of patients and adrenal insufficiency (P-value = 0.46). No significant difference was found when comparing the ages of patients with adrenal insufficiency (P-value = 0.84).
There was no significant relationship between the underlying diseases of diabetes and high blood pressure with adrenal insufficiency in patients (P-value = 0.995 and P-value = 0.685, respectively). By examining vital signs, patients with adrenal insufficiency from the mean systolic and diastolic blood pressures were lower. A significant relationship was observed in comparing the two groups (P- value < 0.001 and P-value = 0.001, respectively).
When we compared the sodium and potassium levels between the two groups, patients with adrenal insufficiency had lower mean serum sodium, in the group with normal adrenal gland function, there was 22.5% hyponatremia and 22.5% hypernatremia. While 75% of patients with adrenal insufficiency had hyponatremia, hypernatremia was not observed in any patients (P-value < 0.001).
In contrast, the two groups had no significant difference in the potassium level (P-value = 0.587). There was also no significant difference in the in-hospital mortality rate in the follow-up of the patients (P-value = 0.322).
Furthermore, there was no considerable discrepancy in the in-hospital mortality rate during the patients' follow-up period (P-value = 0.322).
Based on the study conducted by Juster et al. (23), which involved 204 adults, women had higher cortisol levels in the mornings. This variation was attributed to the influence of sex hormones on the cortisol profile. However, our study found no significant difference in the baseline cortisol serum levels between men and women. This lack of difference could be attributed to the presence of other important factors that impact cortisol levels, such as underlying diseases and levels of stress. These additional factors might have contributed to the observed cortisol variations, highlighting the complexity of cortisol regulation in different populations.
According to Li et al., the virus primarily utilizes angiotensin-converting enzyme 2 (ACE2) as its main entry mechanism, prominently in numerous endocrine glands. Interestingly, their study found no significant difference in ACE2 expression levels between males and females and between younger and older individuals. These findings suggest that the risk of SARS-COV-2 and SARS-COV infections may not be significantly associated with factors such as sex, age, or race (24).
A study conducted by Mao et al. in 2021 found that cortisol levels in critical COVID-19 patients were significantly lower compared to the non-critical group. Specifically, six patients with severe COVID-19 symptoms exhibited cortisol levels of less than ten µg/dl in their plasma, which aligns with our findings (25).
During the critical stage of COVID-19, Alzahrani et al. (26) observed low levels of cortisol (average: 196 nmol/L) and ACTH (average: 18.5 nmol/L) in 28 patients who had not received glucocorticoids and had no other medical conditions that could affect their adrenal functions. In the second round of tests, these results were observed in 60% of cases, indicating a potential secondary adrenal insufficiency. The patients exhibited reduced cortisol and ACTH levels, suggesting a direct relationship between COVID-19 infection and dysfunctions in glucocorticoid responses. This study showed a sudden disturbance in the response of the adrenal cortex among COVID-19 patients by examining cortisol and ACTH levels, which was a sign of adrenal insufficiency in these patients.
Julienne Sânchez et al. published a report detailing the case of a 64-year-old woman presenting with stomach ache, nausea, vomiting, and a positive COVID-19 diagnosis. The patient's tests revealed hyponatremia and a 2.6 µg/dL baseline cortisol level. Notably, after cosyntropin injections, cortisol levels changed to 2.3 µg/dL and 2.9 µg/dL at 30 and 60 minutes, respectively, indicating adrenal dysfunction. After administering hydrocortisone, the patient's hypothermia and hypotension showed improvement (27). These results correspond with our findings.
In a separate report by Hamazaki et al., a COVID-19 patient exhibited a baseline cortisol serum level of 8.2 µg/dL and ACTH levels of 4.9 pg/dL. Adrenal insufficiency was confirmed through CT scans and other diagnostic tests, aligning with our findings (28).
In a study by Iraj Ahmadi et al., cortisol and ACTH levels were measured in 154 COVID-19 patients during the first two days of hospitalization. The average plasma cortisol level was 15.6 µg/dL, and the average ACTH level was 11.4 pg/dL. It was observed that 9.09% of patients succumbed to the illness. Significantly lower baseline cortisol levels (11.3 µg/dL compared to 16.7 µg/dL) were evident in those patients (P value < 0.01). These study findings correspond with our research, emphasizing the importance of timely corticosteroid treatment in reducing mortality rates and effectively managing patients (29).
Ekinci et al. compared 107 patients with mild COVID-19 infections to a control group. The study, focusing on adrenocortical system hormones, found that the baseline cortisol serum level and cortisol/ACTH ratios were significantly elevated in the patients compared to the control group. Conversely, ACTH serum levels were lower, supporting our findings (30).
A separate study by Tan et al. examined the cortisol serum levels of 403 non-critical COVID-19 patients. The average baseline cortisol levels in the COVID-19 patients were found to be 22.43 nmol/L, which was significantly higher than the levels observed in the control group (18.81 nmol/L) (P value < 0.001). These results indicate a heightened cortisol response in these patients, confirming our findings (31).
Earlier investigations on SARS, which emerged in 2003, indicated the potential impact of the disease on various endocrine axes. Given the etiologic and pathologic resemblance between SARS-COV and SARS-COV-2, it is conceivable that COVID-19 may also involve the endocrine system (32). The pathogenesis of endocrine dysfunction in COVID-19 can be attributed to the specific ACE2-mediated viral entry and damage, direct viral toxicity, and the dysregulation of various cytokines, leading to a "cytokine storm" characterized by the release of IL-6, IL-10, IL-1β, IL-17, and TNF-α (1, 9). These mechanisms disrupt the normal functioning of the endocrine system, potentially resulting in adrenal insufficiency.
However, clinical evidence regarding adrenal involvement in COVID-19 patients is currently limited and is confined to case reports and small patient series (1, 16, 27, 28). Our clinical trial study investigated a larger cohort of individuals with COVID-19 to address this knowledge gap. Our findings revealed a significant prevalence of hypercortisolism, indicating adrenal insufficiency, among patients with COVID-19. Therefore, healthcare professionals should consider the possibility of adrenal insufficiency in COVID-19 patients with unexplained manifestations such as hyponatremia and persistent hypotension. Timely administration of corticosteroids may be necessary to manage these patients effectively.
Our study examined the mean baseline and 60-minute serum cortisol levels following the cosyntropin test. The results revealed a significant difference between the baseline and post-cosyntropin cortisol levels (P-value < 0.001). These findings indicate that the cosyntropin test effectively increased serum cortisol levels, indicating a robust response to the cosyntropin stimulation.
This study provides valuable insights into the adrenal glands' functioning and ability to produce cortisol in response to cosyntropin. The significant increase in cortisol levels post-cosyntropin test indicates normal adrenal gland function and responsiveness.
It is important to consider the limitations of our study, such as the sample size and specific population studied. Further research with larger cohorts and diverse populations is needed to validate and generalize these findings.
Overall, our study demonstrated the utility of the cosyntropin test in assessing adrenal gland function and evaluating cortisol production. These results contribute to understanding adrenal health and can inform clinical decision-making in managing patients with adrenal insufficiency or related conditions.
Further research is needed to investigate the underlying mechanisms, long-term implications, and optimal treatment strategies for adrenal insufficiency in COVID-19. Understanding these aspects will empower healthcare providers to offer tailored interventions and enhance patient outcomes in this complex clinical scenario.
4.1. Limitations
Due to the possibility of delaying the treatment of patients with additional tests, we could only examine possible patients with primary adrenal insufficiency. Therefore, many patients with secondary adrenal insufficiency may have been missed.
We excluded patients from the intensive care unit due to the potential influence of other factors on their adrenal functions, such as concurrent infections and administration of inotropic drugs and antibiotics.
4.2. Conclusions
Our study aimed to assess the adrenal gland's response to the inflammatory condition associated with COVID-19 infection. Adrenal insufficiency was observed in approximately 33.3% (20 participants) of the critically ill patients included in the study. These findings underscore the significance of recognizing and evaluating the adrenal axis to enable timely treatment for COVID-19 patients. Prompt identification and management of adrenal insufficiency by healthcare providers can lead to optimized patient care and enhanced outcomes.