1. Context
Temporomandibular disorders (TMDs) are a diverse group of diseases that affect the temporomandibular joint (TMJ), the muscles of mastication, and related structures (1). TMD is the most prevalent chronic orofacial disorder that affects many people (2) and is more prevalent in 20- 40-year-old females (3). The most important clinical signs of TMD include joint sounds, TMJ or muscular pain, and deviations or limitations in mouth opening (4). TMD is typically characterized by pain as the main symptom, and those affected often experience frequent stress in their daily lives (2). The nature of TMD involves multiple factors, but there is debate regarding the specific factors involved (5).
Patients with TMD usually have psychological stress as a significant triggering factor (6, 7). Patients with higher stress are more inclined to bruxism and TMD (8). The significance of psychosocial factors in the etiology of TMD might suggest that these disorders are part of somatoform disorders (9). Therefore, some studies have suggested that the hypothalamic-pituitary-adrenal (HPA) axis plays a role in the pathogenesis of these disorders, and patients with TMD who have inadequate stress responses exhibit increased cortisol secretion (10). Physiological stress activates the HPA axis, which leads to the secretion of cortisol (11, 12), which is then filtered in the acinar cells of the salivary glands and released freely into the saliva (13, 14).
Plasma cortisol is a reliable means to assess the HPA axis, as is salivary cortisol (15). The main advantages of salivary cortisol over serum are the possibility of non-invasive and non-stressful sampling (16). The cause of TMD is not fully understood, but some researchers have explored a potential link between cortisol and TMD. Specifically, they have studied the relationship between salivary cortisol levels and TMD in several different studies (17-25). This systematic review examined the findings from these studies to determine whether there is a correlation between cortisol levels and TMDs.
2. Methods
This systematic review was done according to the PRISMA guidelines (26). The Regional Ethics Committee approved this study (IR.TBZMED.REC.1401.268). The main question was devised according to the “PECO” (population, exposure, comparison, and outcome) approach, where “P” is the whole population, “E” is patients with Temporomandibular joint disorders, “C” is healthy people, and “O” is salivary cortisol levels.
2.1. Eligibility Criteria
Only case-control articles published in English until February 2023 (with no restrictions on publication start dates) were included.
Studies were excluded if they were reprinted articles using information from the same sample, letters to the editor, correspondences, and papers whose full text was unavailable.
2.2. Search Strategy
Electronic research without restriction on publication start date was carried out in October 2022 using four main databases: PubMed, Web of Science, Scopus, and Embase.
All combinations of free and MESH (Medical Subject Heading) terms, including "temporomandibular joint disorders", "temporomandibular joint", "temporomandibular joint dysfunction", "temporomandibular joint disk", "temporomandibular joint disk displacement", "myofascial pain", "myofascial pain and dysfunction”, "myofascial pain disorder syndrome", "myofascial pain dysfunction", "cortisol", "cortisone", "corticosteroid", "salivary cortisol”, "serum cortisol", "blood cortisol", "serum hydrocortisone", "corticosteroid hormone", “hypothalamic pituitary adrenal axis” with “OR” and “AND” operators were searched. The exact search strategy is presented in Appendix 1 in the Supplementary File. The reference lists of the included articles were also searched to identify more papers. The EndNote Basic software was used to identify and remove duplicate references.
2.3. Screening and Selection
The titles were screened by two independent reviewers (H. E. and Z. H.) for compatibility with the research question based on the PECO, and only studies comparing salivary cortisol levels in patients with and without TMD were included. Studies using an intervention, case reports, and studies on patients with fibromyalgia-associated syndromes, sleep apnea, giant-cell arteritis, and autoimmune diseases, such as rheumatoid arthritis, were excluded. Then, the abstracts were reviewed. The authors discussed with the third reviewer (K. K.) whenever there was any disagreement. Then, the full-text articles were examined.
2.4. Data Extraction
After the final selection of studies, the required information was extracted and summarized using a table designed by the Microsoft Excel software.
The first author’s name, year of study, country of origin, type of study, gender of participants, mean age, sample size, type of saliva, and cortisol levels were obtained from the studies (when available) by two independent reviewers (K. K. and Z. H.).
2.5. Assessment of the Risk of Bias
The critical appraisal checklist for case-control studies, developed by the Joanna Briggs Institute (JBI) (27), was utilized by two evaluators (B. A. and Z. H.) independently to appraise the articles. Disagreements were resolved by consulting with the third researcher (H. E.).
2.6. Definitions
TMDs: TMDs refer to a group of clinical conditions that affect the masticatory muscles, TMJs, and related structures. These disorders are characterized by facial pain in the region of the TMJs or muscles of mastication, limitation or deviation in mandibular movements, hyperalgesia of the musculoskeletal structures, and TMJ sounds during jaw movement and function (28).
Unstimulated saliva: The saliva produced at rest, which is saliva secreted most of the day, can be collected by spitting and absorbent methods. In the spit method, the patient allows saliva to collect in the mouth and then spit into a pre-weighed tube, usually every 60 seconds for 5 to 15 minutes. The absorbent method uses a pre-weighed sponge placed in the mouth for a predetermined time. To ensure an unstimulated sample, the patient is instructed to refrain from eating, drinking, smoking, chewing gum, and oral hygiene practices or any other oral stimulation for at least 90 minutes prior to the test session. Excessive movement and talking are discouraged during the test period (29).
Stimulated saliva: Stimulation of salivation typically occurs between 10% and 20% of the day, influenced by olfactory, gustatory, and mechanical stimuli. The secretion of salivary fluid and salivary proteins is controlled by both the sympathetic and parasympathetic subsystems of the autonomic nervous system. Upon stimulation, the volume of the saliva increases, and it becomes more hypotonic and less viscous. Chewing an unflavored gum base or an inert substance, such as paraffin wax or a rubber band, at a controlled rate (usually 60 times per minute) is a reliable and repeatable method of inducing salivation. Also, 2% citric acid may be placed on the tongue at 30-second intervals (30).
2.7. Meta-analysis
The mean difference between salivary cortisol levels was calculated for the studies, which provided the required data. I2 and Q indices were used to measure the heterogeneity between the studies. Also, I2 greater than 50% was considered a significant heterogeneity. The results were combined by the random-effects model using CMA 2.0. A P < 0.05 was considered significant.
3. Results
3.1. Search Results
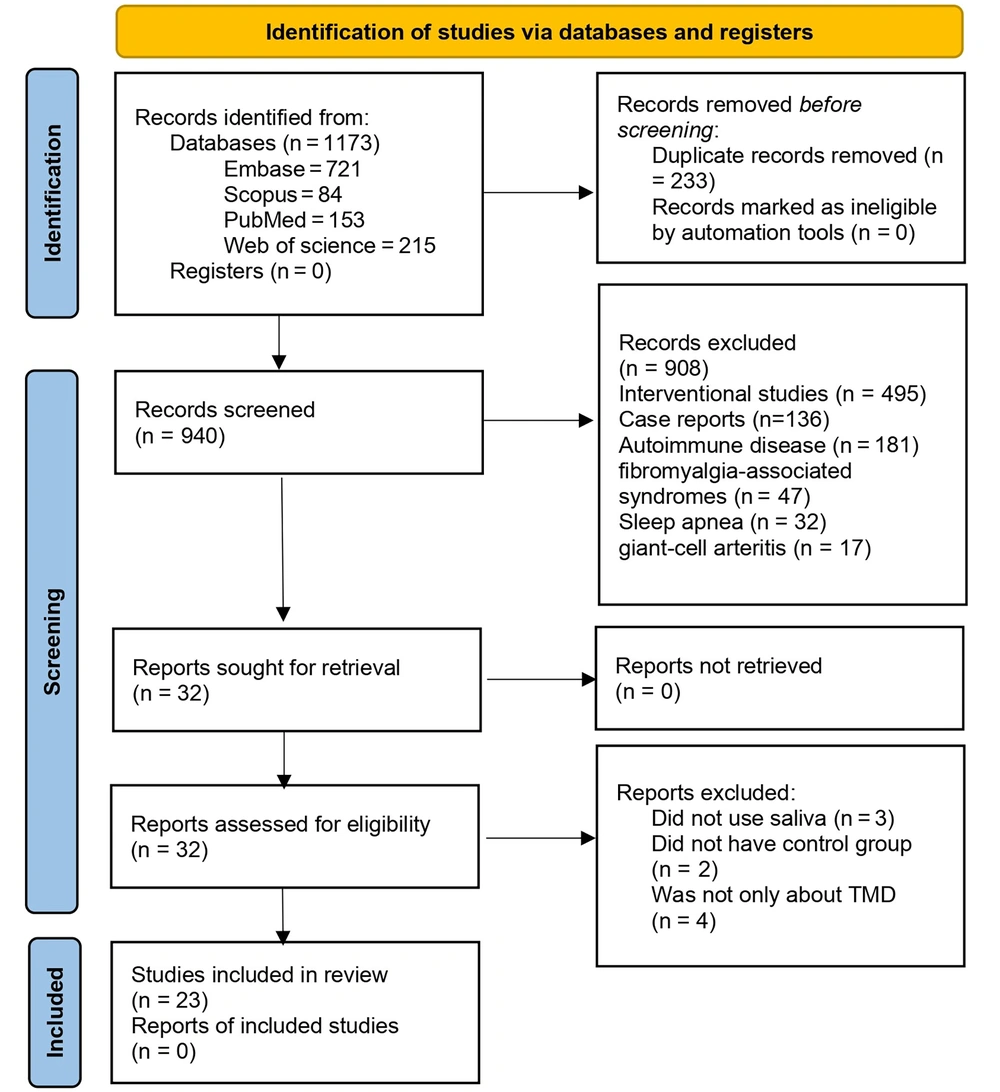
The electronic search in the mentioned databases produced 1173 articles. After duplicate removal, 940 articles remained, from which interventional studies (n = 495), case reports (n = 136), and studies on autoimmune diseases (n = 181), fibromyalgia-associated syndromes (n = 47), sleep apnea (n = 32), and giant-cell arteritis (n = 17) were excluded, and finally, 32 articles were sought for retrieval. Out of the remaining 32 articles, three articles had not used saliva (31-33), two had no control group (34, 35), and four had discussed other diseases in addition to TMD (18, 36-38). Twenty-three articles entered the systematic review, and 18 papers were meta-analyzed. Also, 5 articles did not provide the required data for meta-analysis (17, 39-42). The PRISMA flow diagram presents the search results in detail (Figure 1).
3.2. Assessing the Risk of Bias
Based on the JBI tool, out of 18 studies in the meta-analysis, 13 had a low risk of bias, while the remaining five studies were identified with a moderate risk of bias. (Table 1)
| Author | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Total | Risk of Bias |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Da Silva Andrade et al.(43) | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | 90 | Low |
| Nilsson and Dahlstrom (44) | Y | Y | Y | Y | Y | Y | Y | Y | U | Y | 90 | Low |
| Barabosa et al. (45) | Y | N | Y | Y | Y | Y | Y | Y | U | Y | 80 | Low |
| De Almeida et al. (46) | Y | N | Y | Y | Y | N | N | Y | N | Y | 60 | Moderate |
| Kobayashi et al. (20) | Y | Y | Y | Y | Y | N | N | Y | U | Y | 70 | Low |
| Venkatesh et al. (25) | Y | Y | Y | Y | Y | Y | U | Y | U | Y | 80 | Low |
| Jasim et al. (47) | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 100 | Low |
| Nadendla et al. (21) | Y | Y | Y | Y | Y | Y | N | Y | U | Y | 80 | Low |
| Salameh et al. (24) | Y | Y | Y | Y | Y | Y | N | Y | U | Y | 80 | Low |
| Poorian et al. (22) | Y | U | Y | U | Y | N | N | Y | U | Y | 50 | Moderate |
| Galvão-Moreira et al. (48) | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | 90 | Low |
| Vrbanovic et al. (49) | Y | Y | Y | Y | Y | N | N | Y | Y | Y | 80 | Low |
| Chinthakanan et al. (19) | Y | N | Y | Y | Y | N | N | Y | U | Y | 60 | Moderate |
| Staniszewski et al. (50) | Y | Y | Y | Y | Y | N | N | Y | U | Y | 70 | Low |
| Alresayes et al. (51) | Y | U | Y | Y | Y | Y | N | Y | U | Y | 70 | Low |
| Barakian et al. (52) | Y | U | Y | U | U | N | N | Y | Y | Y | 50 | Moderate |
| Goyal et al. (53) | Y | Y | Y | Y | Y | Y | Y | Y | U | Y | 90 | Low |
| Cheon et al. (23) | Y | N | Y | Y | Y | N | N | Y | U | Y | 60 | Moderate |
Risk of Bias of the Included Studies a
3.3. Characteristics of the Studies
Some studies collected stimulated saliva (20, 25, 43-46), while others collected unstimulated saliva (19, 21-24, 47-53). Table 2 shows the included studies' descriptive characteristics and related data. Overall, 1467 participants were examined in these 18 studies. The publication date of studies ranged from 2008 to 2022.
| Author | Country | Sex (Female %) | Participants’ Age (year) in the Case/ Control Group | Sample Size in the Case/ Control Group | Type of Saliva | Cortisol Levels (µg/dL) in the Case Group (Mean ± SD) | Cortisol Levels (µg/dL) in the Control Group (Mean ± SD) |
|---|---|---|---|---|---|---|---|
| Da Silva Andrade et al. (43) | Brazil | 50 | 22.5/22.8 | 20/20 | Stimulated | 0.27 ± 0.10 | 0.39 ± 0.33 |
| Nilsson and Dahlstrom (44) | Sweden | 100 | 19.7/21.2 | 30/30 | Stimulated | 0.41 ± 0.24 | 0.49 ± 0.36 |
| Barabosa (45) | Brazil | 66 | 11/11 | 86/59 | Stimulated | 0.21 ± 0.16 | 0.16 ± 0.12 |
| De Almeida et al. (46) | Brazil | 75 | 21.8/21.2 | 25/23 | Stimulated | 0.27 ± 0.2 | 0.39 ± 0.33 |
| Kobayashi et al. (20) | Brazil | 63 | 10.6/10.6 | 38/38 | Stimulated | 0.15 ± 0.09 | 0.15 ± 0.09 |
| Venkatesh et al. (25) | India | 53 | 20.5/20.5 | 107/241 | Stimulated | 1.10 ± 0.17 | 0.69 ± 0.16 |
| Jasim et al. (47) | Sweden | 100 | 42.3/45.7 | 27/27 | Unstimulated | 0.20 ± 0.26 | 0.21 ± 0.14 |
| Nadendla et al. (21) | India | 55 | 36.6/36.6 | 20/20 | Unstimulated | 1.7 ± 0.29 | 0.53 ± 0.13 |
| Salameh et al. (24) | Syria | 70 | 29.1/29.1 | 60/60 | Unstimulated | 0.21 ± 0.13 | 0.07 ± 0.04 |
| Poorian et al. (22) | Iran | 54 | 32.4/39.6 | 15/60 | Unstimulated | 2.90 ± 0.52 | 0.88 ± 0.95 |
| Galvão-Moreira et al. (48) | Brazil | 64 | 28.5/28.5 | 39/33 | Unstimulated | 0.10 ± 0.04 | 0.084 ± 0.04 |
| Vrbanovic et al. (49) | Croatia | 100 | 39.3/34.3 | 20/15 | Unstimulated | 0.41 ± 0.28 | 0.24 ± 0.16 |
| Chinthakanan et al. (19) | Thailand | 72 | 22/26.0 | 23/21 | Unstimulated | 0.29 ± 0.12 | 0.22 ± 0.06 |
| Staniszewski et al. (50) | Norway | 86 | 44/46 | 44/44 | Unstimulated | 0.26 ± 0.22 | 0.17 ± 0.13 |
| Alresayes et al. (51) | Saudi Arabia | 88 | 16.3/16.5 | 18/18 | Unstimulated | 0.31 ± 0.03 | 0.05 ± 0.00 |
| Barakian et al. (52) | Iran | NR | NR | 24/36 | Unstimulated | 0.49 ± 0.37 | 0.45 ± 0.20 |
| Goyal et al. (53) | India | 50 | 25.5/23.6 | 40/20 | Unstimulated | 0.36 ± 0.22 | 0.12 ± 0.42 |
| Cheon et al. (23) | Korea | 50 | 28.2/25.9 | 32/34 | Unstimulated | 0.2 ± 0.12 | 0.11 ± 0.03 |
The Characteristics of the Included Studies a
3.4. Meta-analysis
The difference between mean salivary cortisol in the case and control groups:
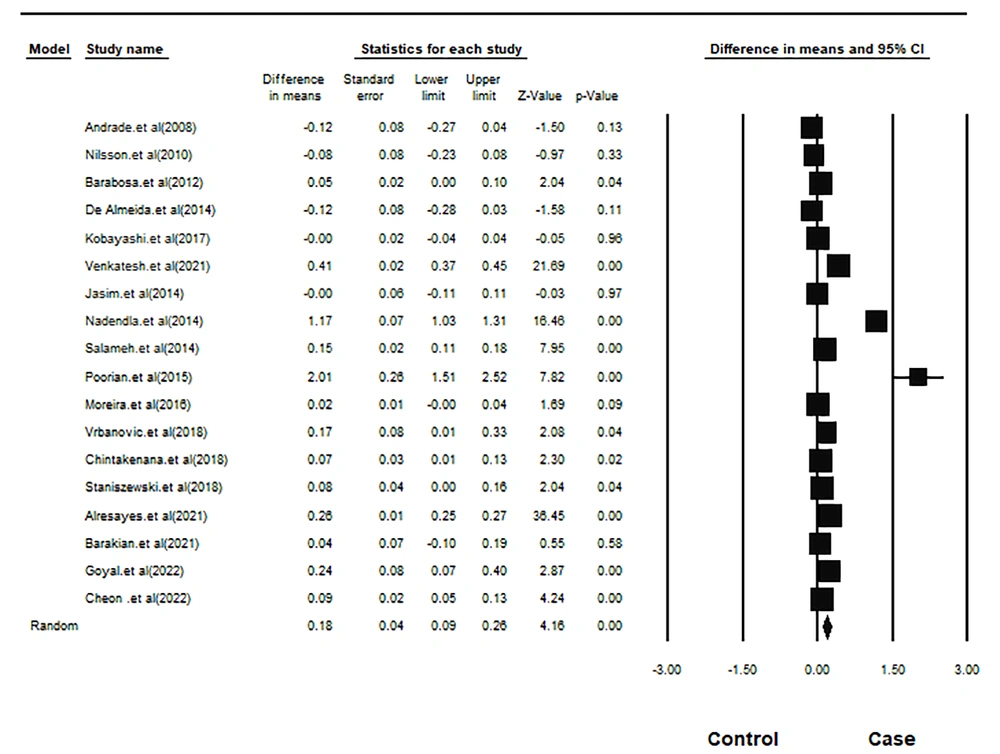
The mean age of 668 participants in the case group was 26.49, and of 799 participants in the control group was 27.03 years. The lack of homogeneity between studies was significant (Q-value = 354.24, df = 17, I-squared = 95.20, and P-value < 0.001). Meta-analysis with the random-effects model indicated that the salivary cortisol in the case group was 0.178 units higher, which was statistically significant (pooled mean difference = 0.178, SD = 0.043, 95% CI = 0.262 - 0.094, z-value = 4.163, and P-value < 0.001). Figure 2 depicts the forest plot of this meta-analysis.
The difference in mean salivary cortisol between study groups based on the type of saliva:
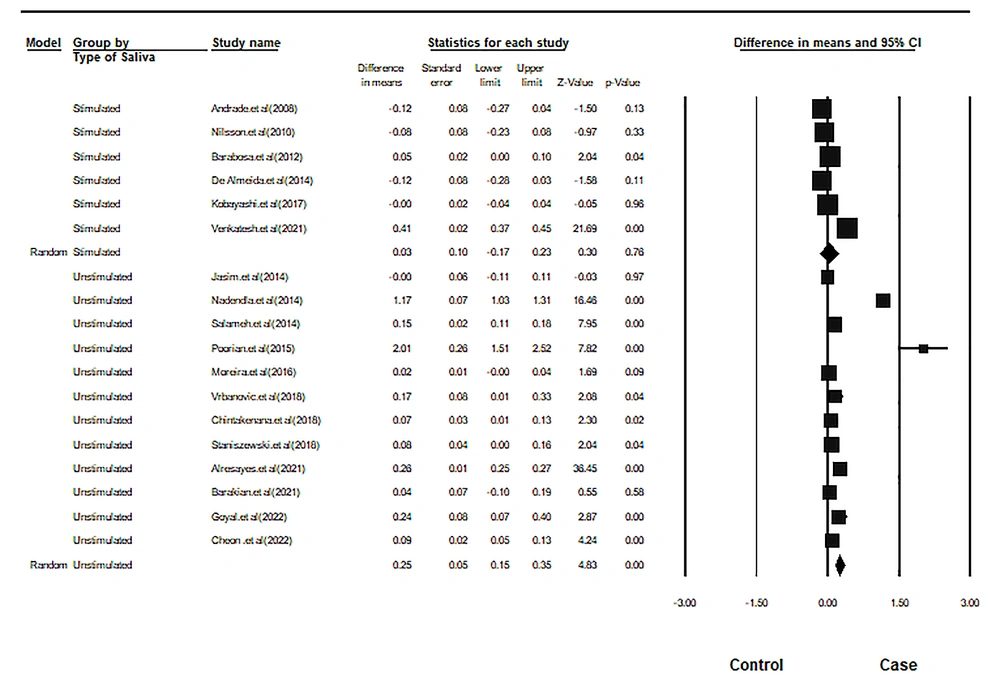
Six studies used stimulated saliva, while 12 used unstimulated saliva. The intragroup difference in the pooled mean salivary cortisol was 0.030 units for stimulated saliva and 0.251 units for unstimulated saliva. This difference was not statistically significant for stimulated saliva but significant for unstimulated saliva. Figure 3 and Table 3 show the results of the subgroup analysis.
| Groups | Effect Size and 95% Confidence Interval | Test of the Null Hypothesis (2-Tail) | Heterogeneity | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of Studies | Point Estimate | Standard Error | Lower Limit | Upper Limit | Z-Value | P-Value | Q-Value | df (Q) | P-Value | I-Squared | |
| Stimulated saliva | 6 | 0.03 | 0.10 | -0.17 | 0.23 | 0.30 | 0.76 | 291.13 | 5 | 0 | 98.28 |
| Unstimulated saliva | 12 | 0.25 | 0.05 | 0.15 | 0.35 | 4.83 | 0.00 | 687.96 | 11 | 0 | 98.40 |
The Results of the Meta-analysis in Subgroups
3.5. Meta-regression Results Based on Age
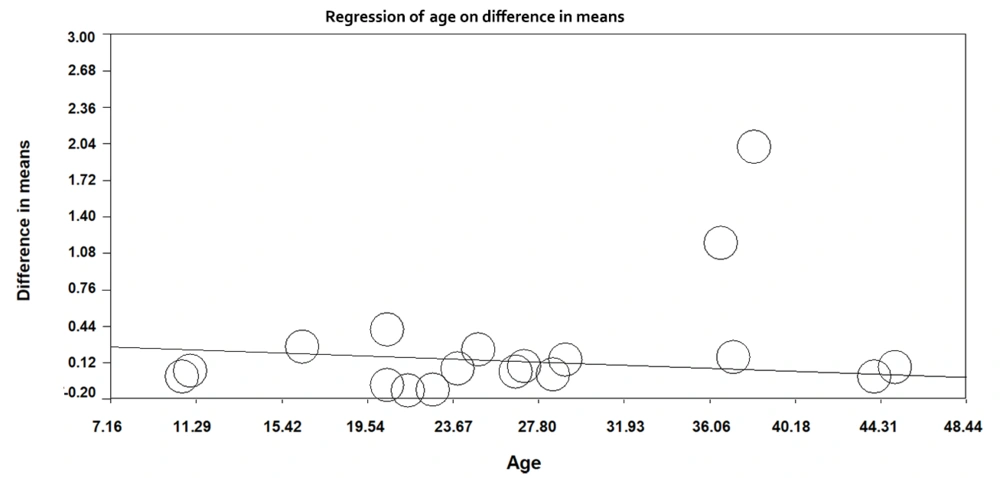
Meta-regression was used to explore the effect of age on the mean difference in salivary cortisol in the case and control groups. With increasing age in the studied subjects, the difference in mean salivary cortisol amongst the two groups decreased by 0.006 units, which was statistically significant (regression slope = -0.006, 95% CI =- 0.0077; -0.0052), P-value < 0.001). Figure 4 shows the meta-regression results.
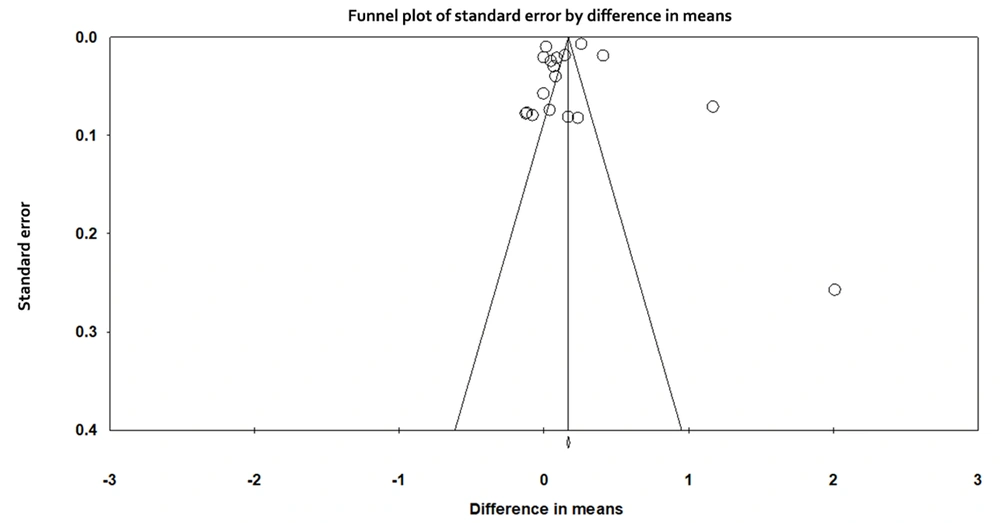
3.6. Publication Bias
The Funnel plot of publication bias is presented in Figure 5. Based on the symmetry in the diagram, there is no publication bias. Also, based on the results of the Egger regression test, the publication bias was not statistically significant (t-value = 0.255, df = 16, and P-value = 0.80).
4. Discussion
According to the results of this systematic review, individuals with TMD exhibited significantly higher salivary cortisol levels compared to the control groups. Salivary cortisol is frequently utilized in research due to its simple collection process and low cost. However, cortisol levels can be affected by the time of collection (54); therefore, only morning cortisol levels were taken into account for this meta-analysis.
In 6 articles that studied stimulated saliva, there was no significant association between the salivary levels of TMD patients and controls, although there were some contradictory results between the studies. Venkatesh et al. showed a significant correlation between salivary cortisol levels and TMD. They suggested that salivary cortisol could be a useful marker to assess the severity of TMD in individuals who experience stress (25). Additionally, Da Silva Andrade et al. showed that females with TMD had higher cortisol levels than the control group; however, there was no significant difference between the two groups in general (43). This could be because males and females respond differently to stress. It is worth noting that in most of these studies, the female-to-male ratio was over 50%, and three studies only included females (44, 47, 49).
In the articles that used unstimulated saliva, salivary cortisol was significantly higher in TMD patients in comparison with controls, although there were inconsistencies between the studies. The differences in the studies could be due to varying types of pain, duration of disease, and study population. Jasim et al. conducted research on salivary cortisol levels in cases of chronic and acute orofacial pain and compared it to a control group consisting of only female patients. The results showed that salivary cortisol levels were not significantly different between the three groups (47). However, Chinthakanan et al. studied TMD patients of both genders who had experienced TMD pain for at least three months. They reported that salivary cortisol levels in the TMD group did not correlate with the visual analog scale (VAS) score (19). This finding is consistent with that of Kobayashi et al., who demonstrated that salivary cortisol levels are not correlated with mild TMD pain, but there is a positive correlation between moderate and severe TMD pain and salivary cortisol levels (20). The pain intensity in the TMD group in the study by Chinthakanan et al. (19) was mild to moderate; hence, no correlation was found between pain and salivary cortisol levels. Thus, it is suggested that salivary cortisol levels are associated with the severity of pain.
The possible explanation is a relationship between the severe pain experienced by the patients and an increase in stress psychobiology, including anxiety and depression. Anxiety can lead to an imbalance of the HPA, and cortisol is the major factor associated with the HPA imbalance (55).
The duration of the TMD could explain why the study results differed. In the study by Vrbanovic et al., patients had experienced pain lasting more than six months (49); therefore, higher cortisol might imply a compensatory increase in the function of the HPA axis. Some articles did not report how long their patients experienced TMD pain.
One of the most frequently suggested mechanisms causing myofascial pain associated with TMDs is hyperactivity of the masticatory muscles (56). When exposed to stress, some patients respond with increased masticatory muscle activity rather than a general increase in body muscle tonus. Such activity manifested as parafunctional habits, can result in muscular fatigue and spasms, leading to myofascial pain and TMD (57).
Bozovic et al. examined 30 university students with myofascial pain and 30 healthy students and found that salivary cortisol levels were higher in students with myofascial pain on both exam days and typical days compared to the control group. Additionally, the salivary cortisol level was found to correlate with psychological factors in students with TMD but not in the control group (17). These findings suggest that preexisting vulnerability, such as TMD, can cause an increase in salivary cortisol levels on stressful days in students with TMD.
As shown in Table 2, the salivary cortisol levels in both stimulated and unstimulated saliva were in a similar range. In general, the concentrations of salivary components that are actively transported, like cortisol, are not significantly reduced in stimulated saliva; however, the concentration of proteins that are not actively transported will tend to decrease in stimulated compared to unstimulated saliva (58).
Higher salivary cortisol levels in TMD patients indicate that stress, either with a role in the development of TMD or pain-related TMD, produces additional stress to the body and further enhances cortisol secretion. For a definite conclusion, longitudinal prospective studies are needed.
Three articles (20, 45, 51) studied children and adolescents, while the others studied adults, which is one of the limitations of this systematic review. Females have higher salivary cortisol levels than men; therefore, gender distribution plays an important role. Seven studies (22, 25, 45-, 51, 52) failed to provide the same female-to-male ratio between the groups, which might contribute to the heterogeneity of the results.
5. Conclusions
This study showed that individuals with TMD have higher cortisol levels in their saliva than those without TMD. This could be due to stress, anxiety, and depression. It is recommended that psychological support be included in the treatment plan for TMD. However, as there are variations in the findings of different studies, further research with a larger sample size and a prospective design would help gain more insight into this issue.