1. Background
Liver cirrhosis is the fibrosis of hepatocytes, ultimately leading to portal hypertension and synthetic liver dysfunction (1, 2). This complex chronic disease causes over 1 million deaths yearly and is recognized as the primary risk factor for hepatocellular carcinoma (HCC) (2, 3). Compensated cirrhosis is often asymptomatic, while decompensated cirrhosis is characterized by complications including portal hypertension, ascites, spontaneous bacterial peritonitis (SBP), hepatic encephalopathy, and bleeding from gastrointestinal varices (4, 5). Disease progression and complications are the leading causes of mortality in these patients (2, 6), with infections such as SBP causing many deaths (7). Hence, identifying markers for predicting cirrhosis complications and prognosis is crucial and contributes to better management strategies (8, 9).
Systemic inflammation commonly occurs in patients with advanced liver cirrhosis (10) and is associated with adverse outcomes (11). The neutrophil-to-lymphocyte ratio (NLR) is a marker of systemic inflammation and highlights the association between two immune pathways. The neutrophil count indicates ongoing (or progressive) inflammation, while the lymphocyte count reflects the activity of immunoregulatory pathways (8, 12). The NLR independently predicts outcomes and mortality in patients with liver cirrhosis with hepatocellular carcinoma (HCC), non-alcoholic fatty liver disease (NAFLD), and liver transplantation (12-15). While this marker can predict mortality in patients with liver cirrhosis independently of the Child-Pugh and MELD scores (16-18), its association with cirrhosis complications has sparsely been examined (8).
Despite the importance of promptly detecting liver cirrhosis complications, limited studies have investigated the role of NLR in predicting such complications.
2. Objectives
This study examined the association between peripheral blood NLR and cirrhosis complications in patients with liver cirrhosis.
3. Methods
3.1. Study Design
This retrospective cohort study was conducted on patients with liver cirrhosis visiting the Gastroenterology Clinic of Imam Khomeini Hospital, Ahvaz, Iran, in 2020. The Research Council and Ethics Committee of Ahvaz University of Medical Sciences approved this study (IR.AJUMS.HGOLESTAN.REC.1400.161). In all the stages of this research, the ethical principles outlined in the Declaration of Helsinki were followed, and patient confidentiality was preserved.
3.2. Sampling and Eligibility Criteria
Convenience purposive sampling was performed. All patients with compensated cirrhosis who aged over 18 years and visited the Gastroenterology Clinic of Imam Khomeini Hospital in Ahvaz in 2020 were included and followed up retrospectively for 1 year regarding cirrhosis complications. The exclusion criteria were suffering from HCC or other non-hepatic malignancies, immunocompromised conditions, sepsis, secondary bacterial peritonitis due to any surgery, overt hypothyroidism or hyperthyroidism, peripheral vascular diseases, major cardiac problems, autoimmune diseases, neoplasms, hematological disorders, unrelated infections that could affect blood white blood cell (WBC) levels, skin infections, and pulmonary infections.
3.3. Data Collection
Baseline demographic and clinical information was retrieved from the patients' medical records, including the cause, duration, and severity of liver cirrhosis. A gastroenterologist diagnosed liver cirrhosis based on liver biopsy, clinical, laboratory, and/or imaging (ultrasonography, endoscopy, FibroScan®) results. The baseline total WBC, lymphocyte, and neutrophil counts were recorded, and the NLR was calculated by dividing the absolute number of neutrophils by the absolute number of lymphocytes.
The severity of liver disease was determined based on the MELD (Model for End-Stage Liver Disease) score and the Child-Pugh score. The MELD score is a valid tool for assessing the severity of liver disease based on international normalized ratio (INR), bilirubin, and creatinine levels. These biochemical parameters are placed in the following formula:
MELD score = 10 × [0.957 × ln (serum creatinine) + 0.378 × ln (serum bilirubin) + 1.12 × ln (INR)] + 6.43
The severity of liver cirrhosis was calculated by the Child-Pugh score based on the presence of ascites, encephalopathy, and bilirubin, albumin, and INR levels. The overall score was classified as class A (5 to 6 points), B (7 to 9 points), or C (10 to 15 points).
The patients were examined for complications of liver cirrhosis, including gastrointestinal bleeding, ascites, hepatic encephalopathy, and SBP over 1 year. Complications were evaluated based on history-taking, clinical examination, laboratory findings, and imaging. Finally, the patients were divided into two groups according to whether or not they developed complications over the 1-year follow-up period, and the variables were compared across the two groups.
3.4. Statistical Analysis
We used SPSS v. 22 (SPSS Inc., Chicago, IL, USA) and MedCalc v. 13 (MedCalc Software Bvba) for statistical analysis. For quantitative variables, the mean and standard deviation (SD) were used to summarize the data, while frequency and percentage were used for qualitative variables. The data distribution normality was checked with the Kolmogorov-Smirnov test. Independent t-tests and the analysis of variance (ANOVA) with post-hoc tests were used to compare the means, and Pearson's correlation and chi-square (or Fisher's exact) tests were used to determine the association between quantitative and qualitative variables, respectively. A significance level of 0.05 was considered.
A receiver operating characteristic (ROC) curve was plotted to evaluate the diagnostic performance of NLR, and the area under the curve (AUC) within a 95% confidence interval (CI) was determined. We evaluated the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) to determine the diagnostic power of peripheral blood NLR to predict the occurrence of cirrhosis complications at the optimal cut-off point.
4. Results
This study involved 256 patients with an average age of 51.56 ± 12.58 years, including 197 men (76.95%) and 59 women (23.05%). The average liver cirrhosis duration was 3.52 ± 2.32 (range: 0.25 - 9) years. Hepatitis B was the predominant cause of cirrhosis, with 85 cases (33.20%).
During the 1-year follow-up, 59 patients (23.05%) developed complications related to liver cirrhosis. The patients who developed complications had a higher average age (P = 0.002). The severity of liver cirrhosis (MELD score and Child-Pugh score), the percentage of peripheral blood neutrophils, and the NLR were also higher in those who developed complications relative to those without complications (P < 0.0001; Table 1).
| Variables | No Complication (N = 297) | Complications (N = 59) | P-Valueb |
|---|---|---|---|
| Age | 49.82 ± 13.42 | 57.36 ± 9.75 | 0.002 |
| Sex | 0.882 | ||
| Male | 152 (77.2) | 45 (76.3) | |
| Female | 45 (22.8) | 14 (23.7) | |
| Cirrhosis cause | 0.249 | ||
| HBV | 62 (31.47) | 23 (38.98) | |
| HCV | 19 (9.64) | 9 (15.25) | |
| ARLD | 37 (18.78) | 7 (11.86) | |
| NAFLD | 56 (28.43) | 18 (30.51) | |
| Autoimmune hepatitis | 14 (7.11) | 1 (1.69) | |
| Other | 9 (4.57) | 1 (1.69) | |
| Neutrophils | 56.63 ± 4.07 | 66.65 ± 4.44 | < 0.0001 |
| Lymphocytes | 38.12 ± 4.60 | 30.17 ± 5.72 | < 0.0001 |
| NLR | 1.51 ± 0.28 | 2.28 ± 0.46 | < 0.0001 |
| MELD score | 14.96 ± 2.02 | 18.81 ± 2.72 | < 0.0001 |
| Child-Pugh score | < 0.0001 | ||
| A | 111 (56.3) | 19 (32.2) | |
| B | 80 (40.6) | 21 (35.6) | |
| C | 6 (3.1) | 19 (32.2) |
Abbreviations: ARLD, alcohol-related liver disease; HBV, hepatitis B virus; HCV, hepatitis C virus; MELD, model for end-stage liver disease; NAFLD, nonalcoholic fatty liver disease; NLR, neutrophil-to-lymphocyte ratio; SD, standard deviation.
a Values are presented as No. (%) or mean ± SD.
b Independent t-test for continuous variables and Fisher's exact test for categorical variables. P < 0.05 was considered significant.
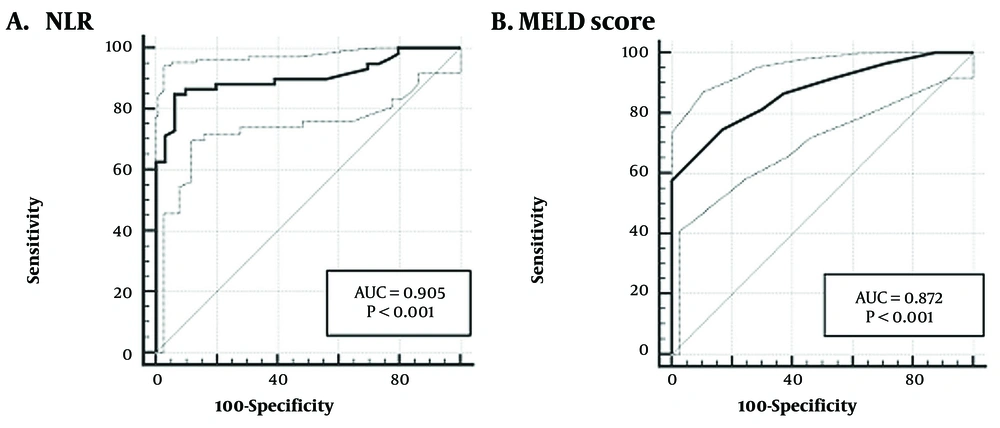
Table 2 demonstrates that the NLR, with a cut-off value of > 1.95, had a sensitivity of 84.75% and specificity of 91.93% for predicting liver cirrhosis complications within 1 year (AUC = 0.905, P < 0.0001). The sensitivity and specificity of the MELD score at the optimal cut-off of > 17 for predicting liver cirrhosis complications within 1 year were 58.74% and 83.25%, respectively (AUC = 0.872, P < 0.0001). The ROC curves depicting the predictive performance of these two parameters are presented in Figure 1.
| Variables | Cut-off (95% CI) | AUROC (95% CI) | P-Value | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) |
|---|---|---|---|---|---|---|---|
| NLR | > 1.95 (1.85 - 2.21) | 0.905 (0.863 - 0.936) | < 0.0001 | 84.75% (73.0 - 92.8) | 93.91% (89.6 - 96.8) | 80.6% (70.4 - 87.9) | 95.4% (91.8 - 97.4) |
| MELD score | > 17 (> 15 - > 18) | 0.872 (0.824 - 0.910) | < 0.0001 | 74.58% (61.6 - 85.0) | 83.25% (77.3 - 88.2) | 57.1% (48.6 - 65.3) | 91.6% (87.1 - 94.4) |
Abbreviations: AUROC, area under the receiver operating characteristic curve; MELD, model for end-stage liver disease; NLR, neutrophil-to-lymphocyte ratio; NPV, negative predictive value; PPV, positive predictive value.
a P < 0.05 was considered significant.
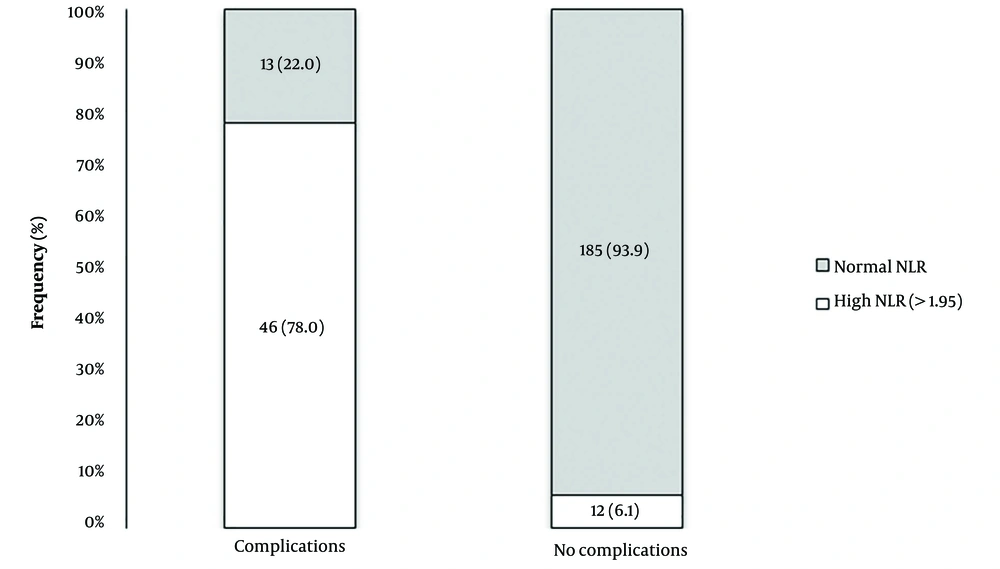
As shown in Figure 2, 78.0% of patients who developed complications had an initial NLR above 1.95, compared with only 1.6% of those who did not (P < 0.0001). Pearson's correlation analysis confirmed a direct and significant association between the NLR and MELD score (r = 0.653, P < 0.0001). A direct and significant association also existed between the NLR and Child-Pugh score (P < 0.0001; Table 3).
| Child-Pugh Score | Mean ± SD | 95% CI for Mean | P-Value a |
|---|---|---|---|
| A | 1.42 ± 0.33 | 1.37, 1.48 | Child-Pugh score A vs. B: < 0.0001 |
| B | 1.88 ± 0.31 | 1.74, 1.88 | Child-Pugh score B vs. C: < 0.0001 |
| C | 2.39 ± 0.42 | 2.19, 2.56 | Child-Pugh score A vs. C: < 0.0001 |
a Analysis of variance (ANOVA) with the post-hoc test.
5. Discussion
During the 1-year follow-up, 23.05% of the patients developed liver cirrhosis complications. The baseline severity of liver cirrhosis (based on the MELD score and Child-Pugh score) and the peripheral blood NLR values were greater in those who developed complications than in those who did not. Our findings indicated that elevations in NLR can predict cirrhosis complications during the subsequent year in patients with compensated liver cirrhosis. At the optimal cut-off value of > 1.95, the NLR had high sensitivity and specificity (84.75% and 91.93%, respectively) in predicting cirrhosis complications, performing better than the MELD score.
Previous studies have shown that NLR is a useful inflammatory marker in various clinical fields, with the ability to predict the prognosis of diseases such as cancer, cardiovascular disease, and type 2 diabetes (19-21). Although most studies associated increased NLR with poor outcomes and prognosis, the exact cut-off value remains debated. In the present study, the average NLR in liver cirrhosis patients who later developed complications was 2.28, compared with 1.51 in those who did not develop complications within the 1-year follow-up period. In the study by Vineeth et al., the average NLR in liver cirrhosis patients was higher than in other studies and equal to 5.82. This could be because their cohort study was conducted on patients hospitalized for the treatment of cirrhosis or its complications, and they did not include outpatients with compensated cirrhosis (6). In the study by Biyik et al., the mean NLR was 2.72, with values higher than the mean being associated with an increased mortality rate (18). Vineeth et al. also linked a rise in NLR with the occurrence of cirrhosis complications, such that 66.7% of the patients with NLR > 12 had more than two complications (hepatic encephalopathy and SBP) (6). Popoiag et al. found that with an optimal cut-off of > 2.4, the NLR had a sensitivity of 98.61% and specificity of 81.94% for predicting the incidence of SBP, as liver cirrhosis patients with and without SBP had mean NLR values of 3.67 and 1.87, respectively. Hence, the NLR can help predict the occurrence of SBP in liver cirrhosis patients (22).
Mousa et al. examined 180 liver cirrhosis patients, demonstrating that the NLR in patients with SBP was significantly higher than in patients without SBP. In that study, NLR values above 2.89 had a sensitivity of 80.3% and a specificity of 88.9% for diagnosing SBP (23). Cai et al. (24) also reported that the average NLR was 2.64 in hospitalized liver cirrhosis patients without bacterial infection and 6.64 in those with bacterial infection, and the NLR with an AUC of 0.824 could be used to predict the incidence of hospital-acquired bacterial infections in patients with decompensated liver cirrhosis. In that study, patients with complications had a higher mean age, MELD score, and NLR than patients without complications (24). These results are consistent with the findings of the present study.
Maccali et al. also directly associated the NLR with the MELD score and other markers of disease severity, highlighting this parameter as an important predictor of adverse outcomes and mortality. In that study, the NLR was higher in patients with bacterial infections than those without bacterial infections (4.95 vs. 3.49) (25). In the study by Kwon et al. (8), the NLR in liver cirrhosis patients with infections was significantly higher than in those without infections (8.3 vs. 4.9), acting to identify patients at risk of poor outcomes and predict 1-month survival. Chiriac et al. also cited the NLR as a cost-effective means of predicting disease outcomes and complications in intensive care unit (ICU) patients with severe liver cirrhosis. The mean NLR in that study was 11.7; those with higher NLR values had greater in-hospital mortalities, bilirubin levels, and Child-Pugh scores, with a higher incidence of ascites, coagulopathy, and other negative outcomes. Moreover, a direct and significant association existed between the NLR and the MELD score (26).
Piotrowski et al. examined 171 liver cirrhosis patients and reported a significant association between NLR and the presence of infection, but the diagnostic accuracy was low (AUC: 0.606, sensitivity 43.4%, and specificity 86%). Nonetheless, the average NLR was significantly higher in patients with an infection (2.45) than in patients without an infection (1.85) (27). Variations in the average NLR across different studies can be related to the diversity in the characteristics of the studied samples. Besides, most studies were conducted on hospitalized liver cirrhosis patients or patients with bacterial infections, which explains the higher average NLR compared to the present study, as we enrolled only patients with compensated liver cirrhosis.
In the present study, the NLR outperformed the MELD score in predicting liver cirrhosis complications. Although both markers indicate mortality in advanced liver diseases (28), Kalra et al. reported that NLR is useful in assessing the risk of death in liver cirrhosis patients with low MELD scores (17). Moreau et al. also showed that the prognostic role of NLR in patients with severe liver cirrhosis is independent of the MELD score (29), which emphasizes the role of inflammation in the poor prognosis of such patients; this factor is missed by classical prognostic scores such as the MELD score. It has also been observed that NLR varies according to the severity of liver cirrhosis (26, 30). Patients with a more severe disease had higher NLR values in the present study. Therefore, according to the results of the present study and similar studies, NLR can be used as a non-invasive, simple, accessible, and accurate prognostic marker to predict the occurrence of complications in patients with liver cirrhosis.
The present study faced certain limitations. In particular, this study was cross-sectional descriptive research with only one treatment center. Moreover, a comprehensive analysis of the patients’ inflammatory status with pro-inflammatory cytokines such as C-reactive protein (CRP), tumor necrosis factor-alpha (TNF-α), and interleukin 6 (IL-6) was not performed due to the lack of routine use of these markers in the studied center. Examining these markers can help explain the mechanisms behind the obtained results. Furthermore, the NLR was checked only once during the initial visit. Ultimately, better results can be obtained by conducting multicenter studies with larger samples.
5.1. Conclusions
The present study revealed that 23% of patients with compensated liver cirrhosis developed complications during 1 year of follow-up. The baseline peripheral blood NLR was significantly higher in those who later developed complications than in those who did not. At the optimal cut-off value of > 1.95, the NLR had high sensitivity and specificity (84.75% and 91.93%, respectively) in predicting cirrhosis complications, performing better than the MELD score. Hence, the NLR can be used as a simple, accessible, non-invasive, and cost-effective prognostic biomarker to help predict the short-term complications associated with cirrhosis and improve the management of these patients.