1. Context
Head and neck squamous cell carcinomas (HNSCCs) are the most common malignancies affecting the oral cavity, pharynx, and larynx (1, 2). Despite advancements in HNSCC treatment, it still exhibits a low 5-year survival rate of approximately 40 percent (3). Many potential biochemical markers associated with head and neck malignancies originate from the methionine cycle. Methionine, a crucial amino acid, is essential for normal human growth and participates in numerous metabolic pathways (4, 5). Homocysteine (Hcy), an intermediate metabolite in the methionine cycle, influences all methyl and sulfur groups involved in bodily metabolism. DNA methylation plays a crucial role in gene expression, thereby affecting phenotype changes (6). Folate is responsible for remethylating Hcy back to methionine (7). Elevated serum Hcy levels are often linked to folate deficiency (8).
Several studies have documented a significant correlation between the occurrence of HNSCC and decreased serum folate (9-16), as well as increased serum Hcy levels (9, 10, 12, 13, 15, 17, 18). In a systematic review encompassing four studies, HNSCC patients exhibited significantly lower serum folate levels compared to controls (19).
Early detection of HNSCC through biomarkers holds promise in preventing disease progression. Hence, this study aimed to explore the association between serum folate and Hcy levels and HNSCC. To the best of our knowledge, no meta-analysis has yet been conducted on these associations.
2. Evidence Acquisition
The research question addressed whether there is a difference in serum folate and Hcy levels between patients with HNSCC and healthy controls.
2.1. Literature Search and Study Selection
To conduct a systematic review, literature searches were performed using MeSH and free terms based on the PECO (Population, Exposure, Comparator, and Outcomes) strategy across multiple databases, including Medline/PubMed, Web of Science, Google Scholar, ProQuest, EMBASE, and Scopus. Additionally, reference lists and citations of included articles were reviewed for further relevant studies. A comprehensive search was carried out, including ProQuest for dissertations, Google Scholar for conference papers, and https://greymatters.cadth.ca/ for gray literature. The search strategy involved various combinations of terms such as folic acid, folate, pteroylglutamic acid, folvite, folacin, vitamin B9, vitamin M, homocysteine, Hcy, 2-amino-4-mercaptobutyric acid, squamous cell carcinoma, head and neck, oral, HNSCC, OSCC, oral tongue, oral cavity, laryngeal, larynx, nasal cavity, nasopharyngeal, hypopharyngeal, mouth, oropharyngeal, salivary gland, lip, cervical tracheal, tracheal, neoplasm, cancer, biomarker.
The inclusion criteria for selecting studies were articles published in English until November 20, 2023, case-control or cohort studies involving newly diagnosed, untreated HNSCC patients with histopathologically confirmed diagnosis and measurement of serum levels of Hcy and folate, and no restriction on age. Exclusion criteria comprised letters to the editors, meta-analyses, or systematic reviews.
2.2. Data Extraction
Following the extraction of articles from the selected databases, two authors (MMV and KK) independently assessed them. Any discrepancies were resolved by a third author (AB). Articles were screened based on title, abstract, and full text, with those meeting the inclusion criteria selected for further analysis. Extracted data included primary author name, location, publication year, study design, sample size of cases and controls, type of sample specimens, serum folate and Hcy levels with standard deviation in HNSCC patients and control groups, and any subgroup data (e.g., smokers or non-smokers), along with confounding factors in each study.
2.3. Assessment of the Risk of Bias
The retrieved articles underwent evaluation by two independent authors (K.K and M.M.V) utilizing the Joanna Briggs Institute (JBI) assessment checklist comprising 10 items. This checklist aimed to assess the methodological quality of the selected articles and identify potential biases in design, conduct, and analysis, such as appropriate case-control matching, measurement reliability, identification of confounding factors, and utilization of suitable statistical analysis (20). Articles with a JBI checklist score of ≥ 7 were deemed high-quality. In instances of disagreement between the two evaluators, a third evaluator (A.B.) was consulted.
2.4. Statistical Analysis
Meta-analysis was conducted based on sample size, mean, and standard deviation. The heterogeneity among studies was evaluated using the I2 index and the chi-square test. Given the significant heterogeneity observed between studies and I2 values exceeding 50%, the random-effects model was employed for the meta-analyses (21). Publication bias was assessed using the Egger linear regression model (22) and the Begg and Mazumdar rank correlation test (23). The pooled mean difference between case and control groups for serum folate and Hcy levels, along with its 95% confidence interval, was utilized to identify associations between serum folate/Hcy and HNSCC occurrence. Statistical analyses were performed using STATA (version 12).
While most studies included in the meta-analysis assessed both smoking and non-smoking patients, the majority did not report serum folate and Hcy levels separately for these subgroups (9, 14, 17). However, most of these studies separated serum folate and Hcy levels between smoker and non-smoker controls (9, 10, 12-16). To approximate the confounding effect of smoking on serum folate and Hcy levels in relation to HNSCC, five meta-analyses were conducted for smoking and/or non-smoking conditions, along with a meta-analysis comparing smoker controls with non-smoker controls.
3. Results
3.1. Summary of Study Search and Characteristics
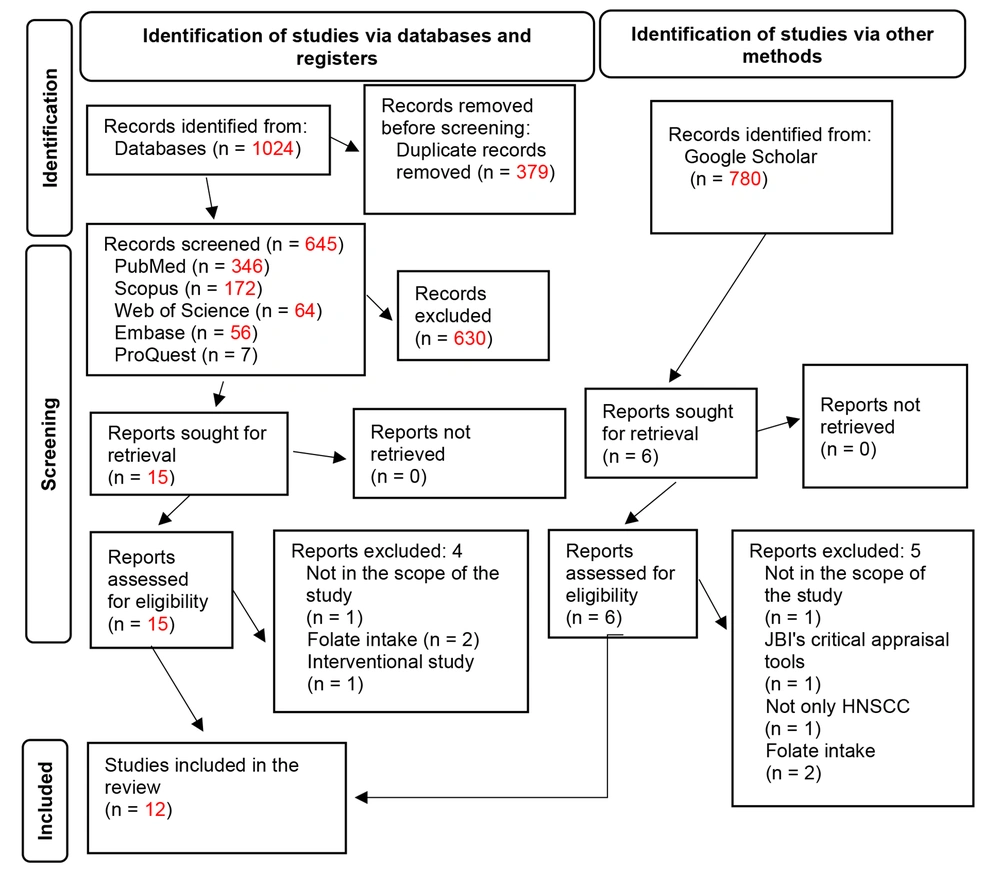
The literature search yielded 1 425 articles after removing duplicate papers within EndNote. Out of the 21 articles that underwent full review, 12 were included in the systematic review. However, only 10 studies were eligible for the meta-analysis, as two studies (18, 24) did not report means and standard deviations. Figure 1 illustrates the search results and screening process.
Of the selected articles, eleven were case-control studies, and one was a cohort study. These articles originated from European (n = 5), Asian (n = 6), and African (n = 1) countries. The types of squamous cell carcinoma cancers studied included comprehensive HNSCC (n = 6), laryngeal (n = 3), and oral cavity (n = 3). The sample sizes varied across the included studies in the meta-analysis, ranging as follows: For the relationship between serum folate levels and HNSCC (493 - 1240 cancer patients; 355 - 1342 controls) and for the relationship between serum Hcy levels and HNSCC (428 - 987 cancer patients; 327 - 1277 controls). A summary of the main characteristics of the included studies in the systematic review and meta-analyses is provided in Tables 1 and 2 for the folate and Hcy studies, respectively. In the majority of studies utilized for the meta-analysis, important confounding factors were either matched between patients and controls or adjusted for.
| Articles | Country | Study Design | Cancer Type | Sample Size (Patients) | Sample Size (Controls) | Folate Level in Patients, ng/mL | Folate Level in Controls, ng/mL | Matched Factors Between Patients and Controls and/or Adjustments |
|---|---|---|---|---|---|---|---|---|
| Akinmoladun and Arinola (18), 2019 a | Nigeria | Case-control | HNSCC | Total: 30; smoker: 19; nonsmokers: 11 | Total: 30; smoker: 2; nonsmokers: 28 | 26.05 (Median) | 30.82 (Median) | Not included in the meta-analysis |
| Chang et al. (11), 2016 | China | Case-control | LSCC | Total: 60; nonsmokers: 60 | Total: 30; nonsmokers: 30 | 3.35 | 4.40 | Matched: No diseases affecting the outcome, no vitamin B intake |
| Erugula et al. (13), 2016 | India | Case-control | OSCC | Total: 30; smokers: 30 | Total: 30; smokers: 15; nonsmokers: 15 | 5.34 | Smokers: 7.68; nonsmokers: 10.99 | Matched: No diseases affecting the outcome, not receiving affecting drugs |
| Fanidi et al. (17), 2015 | 10 European countries | Cohort | HNSCC | Total: 516; smokers: 256; ex-smokersb: 145; nonsmokers: 105; unknown: 10 | 516 (matched control); smokers: 104; ex-smokers: 184; nonsmokers: 214; unknown: 14 | 12.5 | 12.9 (matched control) | Matched: Country, age, gender, not other cancer, date of blood collection |
| Gorgulu et al. (14), 2010 | Turkey | Case-control | LSCC | Total: 60; smokers: 56; nonsmokers: 4 | Total: 60; smokers: 30; nonsmokers: 30 | 5.8 | Smokers: 7; nonsmokers: 7.1 | Matched: Geographic area, age, low to moderate alcohol intake, normal renal function, no hepatic failure, not receiving affecting drugs, no folic acid and vitamin B12 intake, no nutritional deficiency |
| Nacci et al. (15), 2008 | Italy | Case-control | LSCC | Total: 25; smoker: 13; ex-smoker: 12 | Total: 80; smoker: 25; ex-smoker: 30; nonsmokers: 25 | 4.3; smoker: 4.6; ex-smoker: 3.8 | 7.9; smoker: 7.5; ex-smoker: 8; nonsmokers: 8.1 | Adjustment: Age, gender, alcohol intake, cardiovascular disease |
| Eleftheriadou et al. (12), 2006 | Greece | Case-control | HNSCC | Total: 149; smoker: 131 nonsmokers: 18 | Total: 150; smoker: 77; nonsmokers: 73 | 5.32 | Smoker: 5.95; nonsmokers: 8.75 | Matched: Geographic area, age, gender, no systematic alcohol intake, normal renal function, no folate intake |
| Almadori et al. (10), 2005 | Italy | Case-control | HNSCC | Total: 144; smoker: 129; nonsmokers: 15 | Total: 210; smoker: 90; nonsmokers: 120 | 4.87 | Smokers: 9.1; nonsmokers: 9.7 | Matched: Geographic area, age, gender, no habitual alcohol intake, normal renal function, no folate intake, no nutritional deficiency |
| Almadori et al. (9), 2002 | Italy | Case-control | HNSCC | Total: 42; smoker: 39; nonsmokers: 3 | Total: 210; smoker: 90; nonsmokers: 120 | 5.8 | Smoker: 9.1; nonsmokers: 9.7 | Matched: Geographic area, age, gender, low to moderate alcohol intake |
| Raval et al. (16), 2002 | India | Case-control | HNSCC | Total: 214; smoker: 188; nonsmokers: 26 | Total: 56; smoker: 28; nonsmokers: 28 | 11.083; smoker: 10.83; nonsmokers: 12.89 | 11.14; smoker: 11.45; nonsmokers: 10.83 | Adjustment: Age, area, education, income |
Summary of the Characteristics of the Research Included in the Systematic Review and Meta-analysis for the Serum Folate
| Articles | Country | Study Design | Cancer Type | Sample Size (Patients) | Sample Size (Controls) | Homocysteine Level in Patients, μM/L | Homocysteine Level in Controls, μM/L | Matched Factors between Patients and Controls and/or Adjusted for Confounding Factors |
|---|---|---|---|---|---|---|---|---|
| Akinmoladun and Arinola (18), 2019 a | Nigeria | Case-control | HNSCC | Total: 30; smokers: 19; nonsmokers: 11 | Total: 30; smokers: 2; nonsmokers: 28 | 7.84 (Median) | 8.44 (Median) | Not included in the meta-analysis |
| Palaskar et al. (24), 2022 a | India | Case-control | OSCC | Total: 40; smokers: 17; nonsmokers: 23 | 40; nonsmokers: 40 | 18.55 (Median) | 16.85 (Median) | Not included in the meta-analysis |
| Bahramian et al. (25), 2023 | Iran | Case-control | OSCC | 21; nonsmokers | 21; nonsmokers | 3.71 | 2.01 | Matched: Age, gender, normal renal function, no alcohol intake, no diseases affecting homocysteine, no vitamin B12 intake |
| Erugula et al. (13), 2016 | India | Case-control | OSCC | Total: 30; smokers: 30 | Total: 30; nonsmokers: 15; smokers: 15 | 23.58 | Smokers: 17.46; nonsmokers: 10.76 | Matched: No diseases affecting the outcome, not receiving affecting drugs |
| Fanidi et al. (17), 2015 | 10 European countries | Cohort | HNSCC | Total: 516; smokers: 256; ex-smokers b: 145; nonsmokers: 105; unknown: 10 | Total: 516 (matched control); smokers: 104; ex-smokers: 184; nonsmokers: 214; unknown: 14 | 10.8 | 10.2 (Matched control) | Matched: Country, age, gender, not other cancer, date of blood collection |
| Gorgulu et al. (14), 2010 | Turkey | Case-control | LSCC | Total: 60; smokers: 56; nonsmokers: 4 | Total: 60; smokers: 30; non-smokers: 30 | 11.5 | Smokers: 9.7; non-smokers: 8.7 | Matched: Geographic area, age, low to moderate alcohol intake, normal renal function, no hepatic failure, not receiving affecting drugs, no folic acid and vitamin B12 intake, no nutritional deficiency |
| Nacci et al. (15), 2008 | Italy | Case-control | LSCC | Total: 25; smokers: 13; ex-smokers: 12 | Total: 80; smokers: 25; ex-smokers: 30; nonsmokers: 25 | 20.57; smokers: 21.97; ex-smokers: 19.08 | 7.40; smokers: 7.84; ex-smokers: 7.40; nonsmokers: 6.88 | Adjustment: Age, gender, alcohol intake, cardiovascular disease |
| Eleftheriadou et al. (12), 2006 | Greece | Case-control | HNSCC | Total: 149; smokers: 131; nonsmokers: 18 | Total: 150; smokers: 77; nonsmokers: 73 | 9.9 | Smokers: 8.43; nonsmokers: 5.92 | Matched: Geographic area, age, gender, no systematic alcohol intake, normal renal function, no folate intake |
| Almadori et al. (10), 2005 | Italy | Case-control | HNSCC | Total: 144; smokers: 129; nonsmokers: 15 | Total: 210; smokers: 90; nonsmokers: 120 | 13.4 | Smokers: 9.1; nonsmokers: 8.7 | Matched: Geographic area, age, gender, no habitual alcohol intake, normal renal function, no folate intake, no nutritional deficiency |
| Almadori et al. (9), 2002 | Italy | Case-control | HNSCC | Total: 42; smokers: 39; nonsmokers: 3 | Total: 210; smokers: 90; nonsmokers: 120 | 10.4 | Smokers: 8.3; nonsmokers: 7.8 | Matched: Geographic area, age, gender, low to moderate alcohol intake |
Summary of the Characteristics of the Research Included in the Systematic Review and Meta-analysis for the Serum Homocysteine.
3.2. Assessment of the Risk of Bias
According to the JBI tool, out of the 12 included studies, 11 had a low risk of bias, while one study was identified with a moderate risk of bias (Table 3).
| Authors | Q1 a | Q2 b | Q3 c | Q4 d | Q5 e | Q6 f | Q7 g | Q8 h | Q9 i | Q10 j | Total k, % | Risk of Bias l |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Akinmoladun and Arinola (18) | N | N | U | Y | Y | Y | Y | Y | NA | Y | 67 | Moderate |
| Chang et al. (11) | Y | N | Y | Y | Y | Y | Y | Y | NA | Y | 89 | Low |
| Erugula et al. (13) | N | Y | Y | Y | Y | Y | Y | Y | NA | Y | 89 | Low |
| Fanidi et al. (17) | Y | Y | Y | Y | Y | Y | Y | Y | NA | Y | 100 | Low |
| Gorgulu et al. (14) | Y | Y | Y | Y | Y | Y | N | Y | NA | Y | 89 | Low |
| Nacci et al. (15) | Y | Y | Y | Y | Y | Y | Y | Y | NA | Y | 100 | Low |
| Eleftheriadou et al. (12) | Y | Y | Y | Y | Y | Y | Y | Y | NA | Y | 100 | Low |
| Almadori et al. (10) | Y | Y | Y | Y | Y | Y | Y | Y | NA | Y | 100 | Low |
| Almadori et al. (9) | Y | Y | N | Y | Y | Y | Y | Y | NA | Y | 89 | Low |
| Raval et al. (16) | N | Y | Y | Y | Y | Y | Y | Y | NA | Y | 89 | Low |
| Palaskar et al. (24) | Y | N | Y | Y | Y | Y | N | Y | NA | Y | 78 | Low |
| Bahramian et al. (25) | Y | Y | Y | Y | Y | Y | Y | Y | NA | Y | 100 | Low |
Risk of Bias of the Included Studies in the Systematic Review
3.3. Meta-analysis for Folate
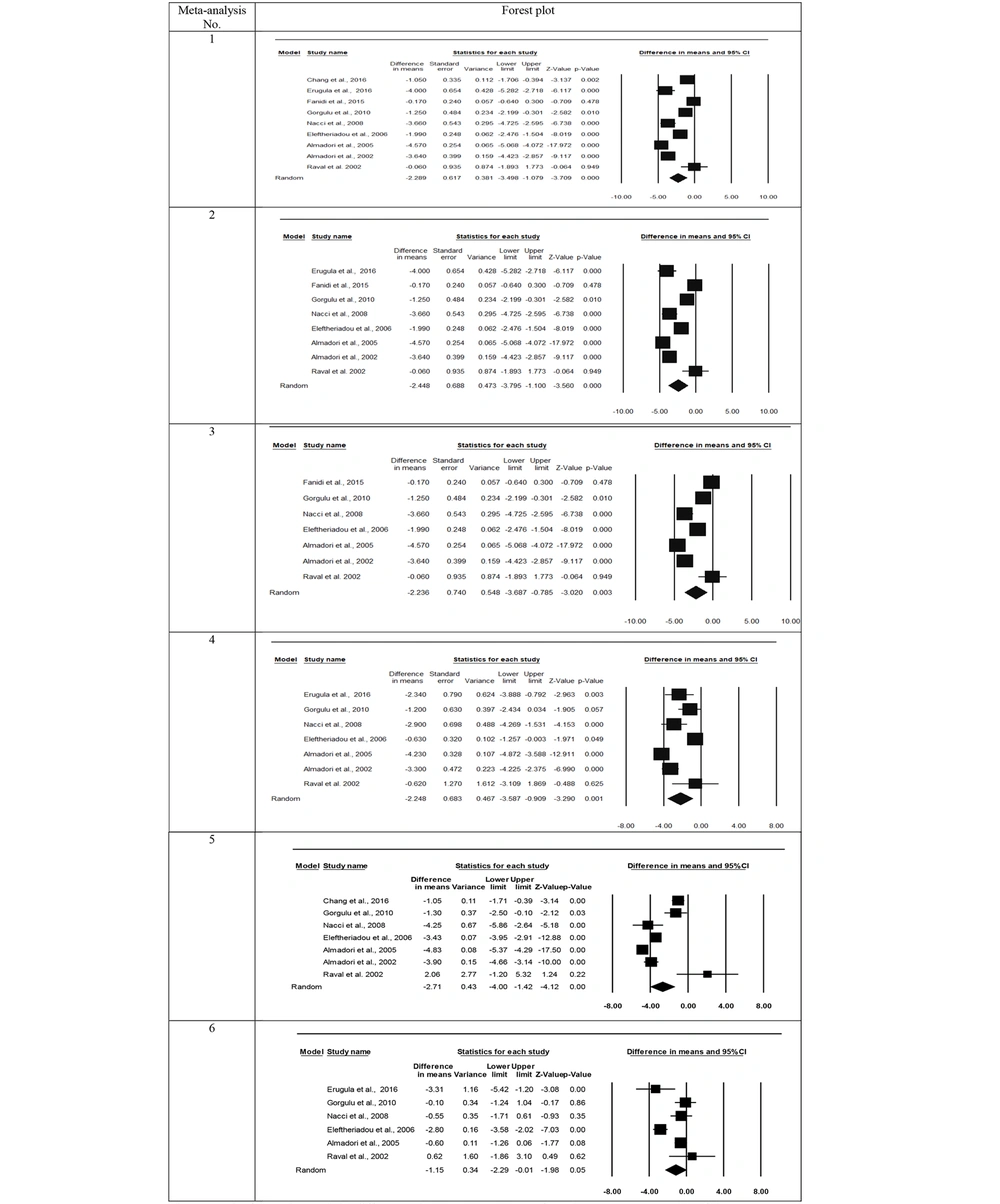
The results of various meta-analyses are presented in Table 4 and Figure 2. The number of studies included ranged from 6 to 9. In all meta-analyses concerning serum folate, the heterogeneity test yielded significant results (Q = 30.0 - 208.0, df = 5 - 8, P-value < 0.001, I2 = 83.3 - 96.8). Consequently, the outcomes were reported using the random effects model. The pooled mean differences between patients and controls across the studies ranged from -2.24 to -2.71 ng/ml of folate, all of which were statistically significant (P-value 0.003 - < 0.001). The negative signs in all analyses indicate that the serum folate level in the HNSCC patient group was lower than that in the control group. Meta-analysis No. 6 (as depicted in Table 4 and Figure 2) compared smoking controls with non-smoking healthy individuals, revealing significantly lower serum folate levels in the smoking controls compared to the non-smoking controls (mean difference = -1.15 ng/mL, P-value = 0.05). All meta-analyses suggested no indication of publication bias, as the Egger test and Begg and Mazumdar test results were not significant (Table 4). The forest plots regarding folate from the meta-analyses are illustrated in Figure 2.
| No. | Meta-analysis | Number of Studies | Heterogeneity Test | Publication Bias | |||
|---|---|---|---|---|---|---|---|
| Q a | P-Value | I2b (%) | Egger Test (P-Value) | Begg and Mazumdar Test (P-Value) | |||
| 1 c | Patients vs. controls (with no regard to smoking conditions in both groups) | 9 | 208.0 | < 0.001 | 96.2 | 0.80 | 0.68 |
| 2 d | Patients vs. controls (smokers or smokers + non-smokers in both groups) | 8 | 194.9 | < 0.001 | 96.4 | 0.80 | 1.00 |
| 3 e | Patients vs. controls (smokers + non-smokers in both groups) | 7 | 188.2 | < 0.001 | 96.8 | 0.95 | 0.88 |
| 4 f | Patients vs. smoker controls | 7 | 71.6 | < 0.001 | 91.6 | 0.82 | 0.65 |
| 5 g | Patients vs. non-smoker controls | 7 | 100.9 | < 0.001 | 94.1 | 0.39 | 0.65 |
| 6 | Smoker controls vs. non-smoker controls | 6 | 30.0 | < 0.001 | 83.3 | 0.88 | 0.57 |
Results of Different Meta-analyses about the Relationship of Serum Folate Levels with Head and Neck Squamous Cell Carcinoma
3.4. Meta-analysis for Homocysteine
The types of meta-analyses for serum Hcy were similar to those for serum folate (Table 5 and Figure 3). The number of studies included in different meta-analyses for Hcy ranged from 6 to 8. All meta-analyses for serum Hcy levels were conducted using the random effects model because all heterogeneity tests were significant (Q = 19.7 - 145.7, df = 5 - 7, P-value < 0.001, I2 = 74.7 - 96.2). The differences between the means of HNSCC and control groups for serum Hcy, pooled over studies, were significant in the meta-analyses (P-value < 0.001) and ranged from 3.56 to 5.60 μM/L. This indicates that the serum Hcy level in the cancer group was higher than in the control group. Although the highest mean difference belonged to the meta-analysis in which HNSCC patients (either non-smokers or non-smokers + smokers) were compared with the non-smoker controls (5.60 μM/L), in meta-analysis No. 10 (Figure 3), the serum Hcy level of patients (either smokers or smokers + non-smokers) was also significantly higher than that of the smoker controls (3.56 μM/L). Additionally, based on meta-analysis No. 12 in Figure 3, smoking controls had significantly higher serum Hcy levels than non-smoking healthy individuals (mean difference = 1.17 μM/L, P-value = 0.02). According to the Begg and Mazumdar test, all meta-analyses showed no indication of publication bias. Also, the Egger regression tests were not significant, except for meta-analysis No. 7 in Table 5 (P-value = 0.047). The forest plots from the meta-analyses regarding Hcy are displayed in Figure 3.
| No. | Meta-analysis | Number of Studies | Heterogeneity Test | Publication Bias | |||
|---|---|---|---|---|---|---|---|
| Q a | P-Value | I2b (%) | Egger Test (P-Value) | Begg and Mazumdar Test (P-Value) | |||
| 7 c | Patients vs. controls (without regard to smoking conditions in both groups) | 8 | 145.7 | < 0.001 | 95.2 | 0.047 | 0.62 |
| 8 d | Patients vs. controls (smokers or smokers + non-smokers in both groups) | 7 | 141.6 | < 0.001 | 95.8 | 0.06 | 0.45 |
| 9 e | Patients vs. controls (smokers + non-smokers in both groups) | 6 | 131.8 | < 0.001 | 96.2 | 0.10 | 0.57 |
| 10 f | Patients vs. smoker controls | 6 | 23.6 | < 0.001 | 78.8 | 0.06 | 0.19 |
| 11 g | Patients vs. non-smoker controls | 6 | 72.9 | < 0.001 | 93.1 | 0.45 | 0.35 |
| 12 | Smoker controls vs. Non-smoker controls | 6 | 19.7 | 0.001 | 74.7 | 0.73 | 0.19 |
Results of Different Meta-analyses about the Relationship of Serum Levels of Homocysteine with Head and Neck Squamous Cell Carcinoma
4. Discussion
Conflicting reports exist in the literature regarding the relationship of HNSCC with serum folate and Hcy levels. Most studies showed a significant association of HNSCC with folate (9-15, 17, 18) and Hcy (9, 10, 12, 13, 15, 17, 25). However, in others, no significant relationship of HNSCC with folate (16) and Hcy (14, 18, 26) was reported. The authors will discuss the results of different meta-analyses for folate and Hcy to verify the existence of an association between HNSCC and serum folate and Hcy levels.
4.1. Folate
The results of different meta-analyses showed significant differences between HNSCC patients and healthy controls. In meta-analysis No. 1, when HNSCC patients were compared with the control group without considering the group's smoking conditions, the serum folate level in patients was 2.29 ng/mL lower than in healthy individuals. The results did not considerably change when one or two studies with different smoking conditions were excluded from the meta-analysis; the mean difference ranged from -2.24 to -2.71 ng/mL. These results indicate the significant association of serum folate level with HNSCC.
Meta-analysis No. 6 (Table 3 and Figure 2) revealed that smoking controls had significantly lower serum folate levels than non-smoking healthy individuals (mean difference of -1.15 ng/mL). Some substances in tobacco smoke interact with folate and lower serum levels in smokers (27). Although the serum folate level in the smoking controls was lower compared to the non-smoking control samples, the magnitude of this difference was lower than in cases when patients were compared with either smoking, non-smoking, or smoking + non-smoking controls (ranging from -2.24 to -2.71). Therefore, the higher reduction in the serum folate level of the HNSCC patients couldn’t be attributed to the smoking conditions alone, and cancerous patients had lower folate than the controls, especially smoking controls, with a mean difference of -2.25.
The main risk factors for HNSCC are smoking (1, 28-36), alcohol (1, 30-32, 35, 37, 38), aging (1, 31), human papillomavirus infection (39, 40), and genetic factors (41), which may have contributed to the reduced folate levels and the onset of HNSCC. However, smoking is regarded as the primary risk factor for HNSCC. In a review study, Hashibe et al. (30) concluded that smoking accounts for 70% of HNSCC patients. In another review article, Whiteman and Wilson (38) reported that smoking contributed to a very high median population attributable fraction (PAF) of > 50% as the epidemiological measure for larynx cancer, and alcohol contributed to a high median PAF of 25 - 50% for oral cavity, pharynx, and larynx cancers.
As mentioned earlier, the serum folate level in HNSCC patients was significantly lower than in both smoking and non-smoking controls. Folate mediates one-carbon metabolism and plays a critical role in several pathways, such as DNA synthesis, amino acid homeostasis, and antioxidant generation, and its deficiency adversely alters these pathways (42, 43). DNA methylation modifies gene expression and transmits epigenetic information through DNA replication and cell division (42). Folate deficiency may result in abnormal methylation of DNA, consequently altering the expression of cancer suppressor genes (44). Additionally, low folate conditions may stimulate uracil production and decrease thymidine synthesis in the DNA sequence during cell division (42, 45), increasing the frequency of chromosomal breaks and, presumably, the risk of carcinogenesis (45).
4.2. Homocysteine
The trend of differences between HNSCC patients and healthy controls for Hcy was similar to serum folate. When HNSCC patients were compared with controls without considering the smoking conditions in both groups (meta-analysis No. 7), the serum Hcy level was 4.73 μM/L higher in the cancerous patients compared to the controls. After excluding one or two studies with different smoking conditions, the results didn’t change drastically, and the difference between patients and controls ranged from 3.56 to 4.49 μM/L Hcy. The lowest mean difference for Hcy (3.56 μM/L) belonged to meta-analysis No. 10, where the patients, smokers or smokers + non-smokers, were evaluated against the smoker controls. However, when non-smokers or non-smoker + smoker patients were compared with non-smoker controls, the mean difference between the two groups for Hcy rose to 5.60 (meta-analysis No. 11). These results also show the significant association of serum Hcy with HNSCC, without considering the smoking conditions of the control groups. Although smoking controls also had significantly higher Hcy levels than non-smoking individuals (mean difference = 1.17 μM/L), this difference was much lower than in the analysis where HNSCC patients were evaluated against healthy controls, regardless of the smoking situation (ranging from 3.56 to 5.60 μM/L). Thus, similar to the findings for serum folate level, the increase in Hcy level in cancerous patients couldn’t be attributed solely to the smoking habit, and HNSCC patients had higher Hcy than the controls (especially the smoker controls).
Folate acts as a methyl donor in the methionine cycle, while Hcy serves as an intermediate metabolite within this cycle (15, 46). Hcy metabolism is crucial for regulating methionine availability and DNA methylation. It is synthesized from methionine through two cofactors: S-adenosylmethionine and S-adenosylhomocysteine. The level of Hcy is maintained through the remethylation pathway, which converts Hcy to methionine, and the transsulfuration pathway, which converts Hcy to cysteine (47). Consequently, alterations in Hcy metabolism lead to hyperhomocysteinemia, which is associated with increased free radicals, induced oxidative stress, and possibly increased risks of cancers and other diseases (13, 48). One reason for the elevation in Hcy levels in HNSCC patients might be the reduction of serum folate, which affects Hcy metabolism (49, 50). Thus, the positive relationship between Hcy and HNSCC may be linked to folate deficiency, resulting in the accumulation of Hcy in the blood serum (17).
4.3. Folate and Homocysteine as Possible Biomarkers
Despite advancements in the treatment of HNSCC through surgery, radiotherapy, and chemotherapy, the disease still carries a low overall 5-year survival rate of about 40 percent (3, 51). Clinical examinations and biopsies often fail to detect HNSCCs, such as oral squamous cell carcinoma, in the early stages (52). Therefore, early diagnosis of these malignancies can reduce morbidity and mortality. Identifying biomarkers would be beneficial in the early detection of these cancers (13, 53). Although Almadori et al. (10) stated that in patients with HNSCC, Hcy levels may not solely depend on folate levels but possibly are influenced by the heterogeneity of the HNSCC phenotype, our meta-analysis of included articles yielded similar results for serum folate and Hcy, both of which showed a significant relationship with HNSCC. Therefore, these biomarkers may prove useful in the early detection of HNSCCs.
There were some limitations in this research. The heterogeneity among the studies concerning confounding factors related to HNSCC patients and controls may have influenced the results.
4.4. Conclusions
The results of different meta-analyses in this study showed that the serum folate and Hcy levels of HNSCC patients were significantly lower and higher, respectively, than those of the control groups, regardless of the smoking condition in both groups. Although serum folate was significantly lower and serum Hcy was significantly higher in the smoking control groups compared to the non-smoking control groups, the magnitude of these differences was smaller when patients were compared with the healthy groups. In conclusion, our meta-analyses suggest a potential association of serum folate and Hcy levels with HNSCC. Therefore, these biochemical compounds may serve as biomarkers for the early detection of HNSCC onset in patients.