1. Background
Tooth caries are among the most common diseases worldwide, and traditional dental treatments, such as endodontic therapy, restorations, and the replacement of missing teeth with prosthetics or implants, have certain disadvantages, often failing to fully restore the ideal esthetics and function of a natural tooth (1). The ability to regenerate a natural tooth would be a significant breakthrough, and stem cell research represents a promising step toward achieving this goal.
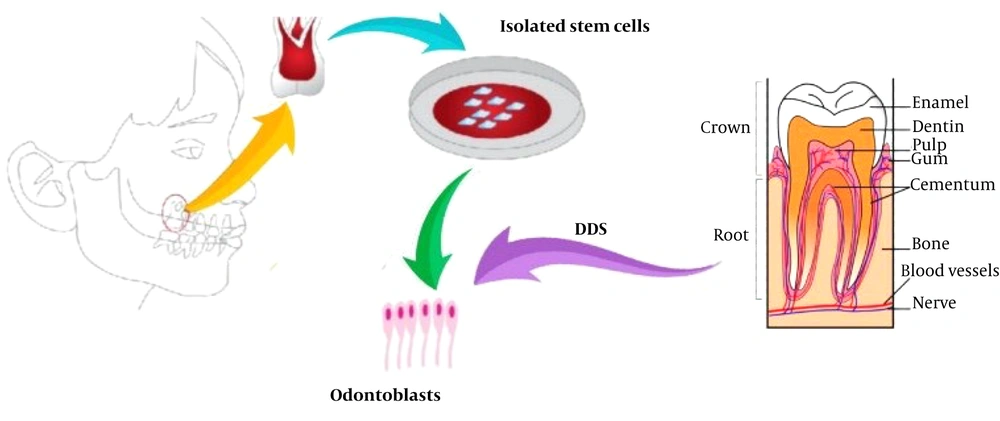
Stem cells have ushered in a new era in regenerative medicine and dentistry. Numerous studies have focused on the isolation, proliferation, and differentiation of dental stem cells to develop methods for regenerating lost tooth tissue (2). Since the pulp of extracted teeth contains dental pulp stem cells (DPSCs), it is one of the best options for dental tissue engineering purposes (Figure 1) (3). To date, dental stem cells have been used to produce dentin-pulp complexes, periodontal fibers, and even bone tissue (4, 5). Previous studies have evaluated various signals and scaffolds for their effects on dental tissue regeneration.
2. Objectives
This study aimed to investigate the role of a self-administered dentin matrix, referred to as dentin derivative signal (DDS), as a factor in the differentiation of DPSCs into odontoblasts.
3. Methods
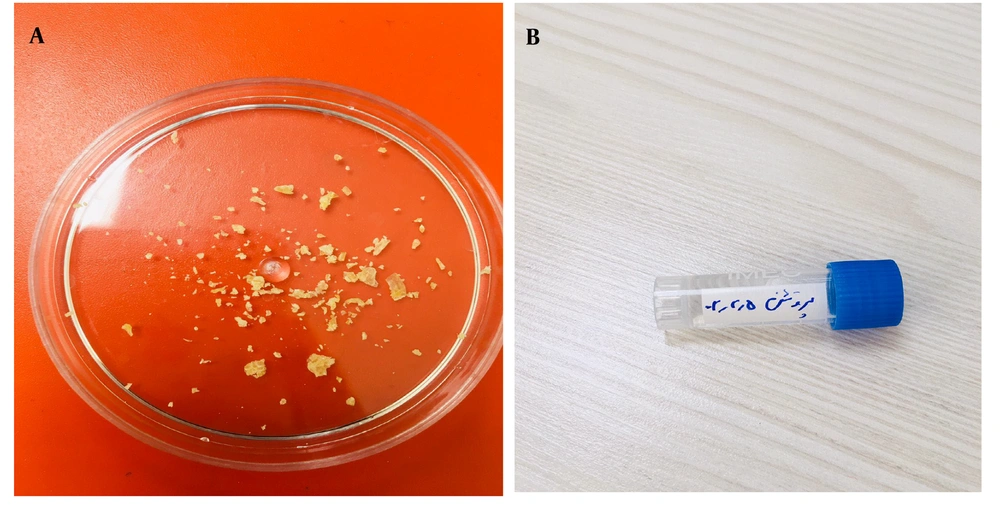
Five extracted teeth used in this study were first disinfected with sodium hypochlorite. Initially, the tissues covering the surface of the teeth, including enamel and cementum, were removed, along with the pulp. The remaining dentin tissue was then shaped into 1 × 1 mm block and soaked in 10% nitric acid for a week (7 days). This process was continued until demineralization had occurred, leaving behind only the protein matrix. Finally, the protein gel was converted into powder using a freeze dryer (Cryodesiccation/Lyophilization) (Christ Co, Germany, Model: Alpha 1-2 LD Plus).
Human dental pulp stem cells (hDPSCs) (DPS-13 cell line with C10896 IBRC cell code) were placed in a 25 mL flask containing α-MEM (Sigma M0644) and ES-FSC (Gibco 16141-079) and incubated at 37°C with 95% humidity and 5% CO2. The cell culture media were exchanged every 2 - 3 days for about two weeks to achieve an appropriate cell density (Figure 2). Cells aggregated on the floor of the flask and were counted using trypan blue and a hemocytometer slide.
Flasks containing hDPSCs colonies (200,000 cells), scaffold-free culture medium, and DDS, which contained the necessary growth/differentiation factors for dentin regeneration (0.7 mL from 100-μg/mL), were prepared for further experimentation.
4. Results
After 3, 7, and 10 days, the cultures were evaluated for cell viability using the 3-[4, 5-dimethylthiazol-2-yl]-2, 5-diphenyl tetrazolium bromide (MTT) method, and for odontoblast differentiation and dentin tissue expression using the immunocytochemistry (ICC) method. The mean ± standard deviation (SD) of the MTT results for the three groups were 0.13 ± 0.03, 0.12 ± 0.01, and 0.10 ± 0.02, respectively, which were not statistically significant (P = 0.465). Thus, the cell viability rate was similar across all groups. Data were analyzed using SPSS version 24, employing the Shapiro-Wilk test, one-way variance analysis, and Tukey test (P-value ≤ 0.05).
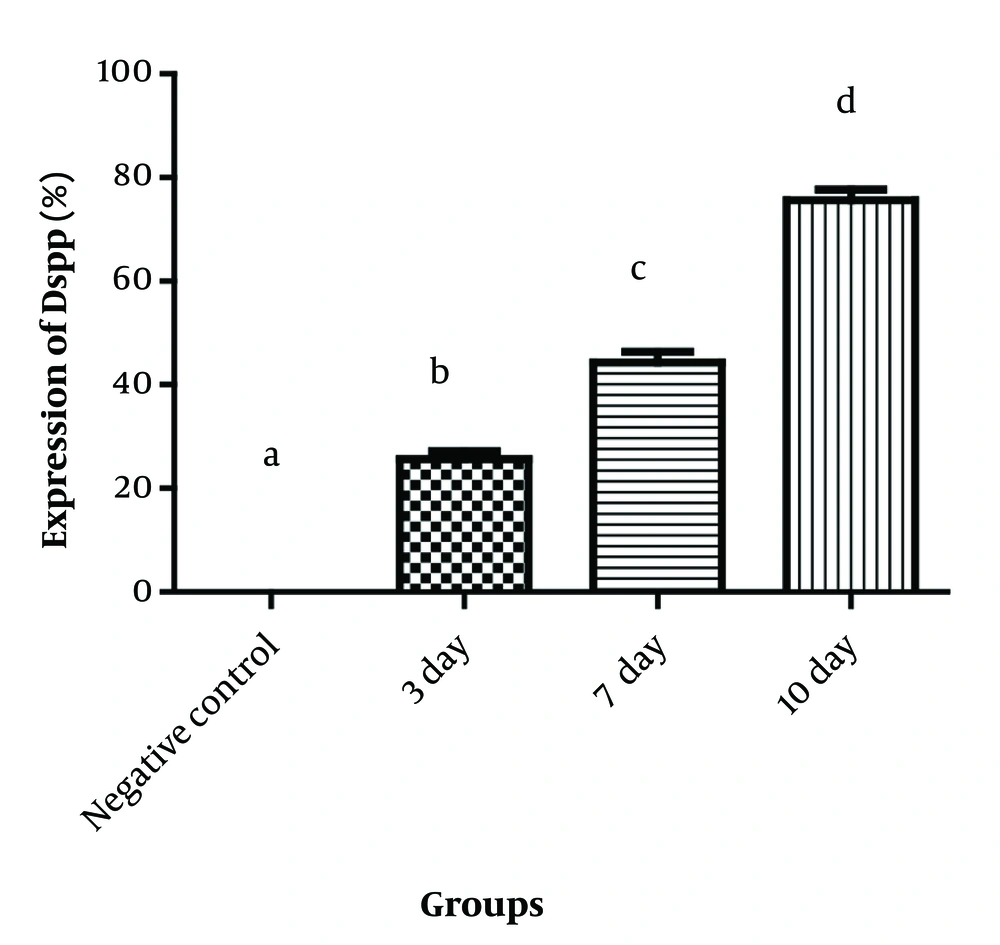
The ICC results showed that the expression percentage of dentin sialophosphoprotein (DSPP), an odontoblast differentiation marker, was significantly higher in the case groups compared to the negative control group, which contained only hDPSCs without any signals (P < 0.001 in all cases). Additionally, there was an increased expression of DSPP on days 7 and 10 compared to day 3, indicating that a longer exposure period resulted in greater dentin expression (Figure 3).
Therefore, the powder of the self-administered DDS preserved the properties of the extracellular matrix of natural dentin. This novel signal, referred to as DDS, has been granted an Iranian patent certification with the number 106171.
5. Discussion
Advancements in regenerative therapy utilizing various stem cells have led to numerous clinical studies, with mesenchymal stem cells being among the most widely used in this era. Dental and, more recently, oral tissues have been recognized as valuable sources of human mesenchymal stem cells. Stem cells derived from dental tissue have been employed in animal studies and clinical applications due to their multipotential differentiation, ease of accessibility, and non-invasive preparation methods, making them suitable for in vitro studies as well. As demonstrated in this study, dental pulp-derived cells possess significant potential to differentiate into all dental tissues, acting as multipotential stem cells for tooth regeneration.
Yamada et al. evaluated 815 clinical trials involving dental pulp-derived cells for bone regeneration, periodontitis, and dental pulp regeneration. Their findings revealed that these stem cells are promising candidates for further medical research in the field of regeneration (6). Dental pulp stem cells exhibit considerable differentiation potential, capable of transforming into various tissues, including mesodermal adipogenic and chondrogenic lineages or ectodermal lineages (7). In addition to these potentials, they can differentiate into odontogenic and osteogenic tissues (8-10), as well as periodontal and other dental tissues (11, 12).
Defects in the jaws caused by tooth loss or pathological lesions are common and have significant morbidity. Therefore, emerging regenerative therapies offer hope to patients, enabling them to lead better and more fulfilling lives. Mesenchymal stem cells have the potential to differentiate into bone and dental tissues, leading researchers to seek growth/differentiation factors as inducing signals for these cells (13).
Incorporating commercial technologies into scientific advancements will make treatments more accessible to patients worldwide. Consequently, regenerative dentistry needs to be commercialized, similar to other fields of dental research. Recent research in this field has focused on identifying more suitable signals for stem cell induction, thereby increasing patient access to this technology (14).
Several growth or differentiation factors (signals) have been the subject of recent research, including basic fibroblast growth factor (bFGF), which has been reported to induce cell proliferation, migration, and differentiation. Previous studies have identified bFGF in DPSCs, and their receptors FGFR1 and FGFR2 are also expressed. Exposing DPSCs to bFGF has been shown to lead to proliferation and differentiation during neurogenesis and osteogenesis. In some studies, functional repair was observed in rats treated with DPSCs and bFGF, although other studies indicated that the effect on DPSCs could be time-dependent. Overall, most studies suggest that bFGF promotes DPSC proliferation into neurogenic and osteogenic tissues, making it a potential candidate for generating lost bone and nerve tissue in pathological or traumatic events in the head and neck region (15).
Another important growth factor is transforming growth factor-β (TGF-β), which is involved in development, hematopoiesis, wound healing, carcinogenesis, and immune responses. This factor has shown effects on specific cell types, including DPSCs and bone marrow-derived MSCs (BM-MSCs). When activated, TGF-β can influence stem cell migration, proliferation, differentiation, and apoptosis. Dental pulp stem cells, in particular, are responsive to this growth factor and can be induced to differentiate into other cell types (15). Several studies have demonstrated that treatment with recombinant TGF-β1 can lead to dental pulp cell proliferation. For instance, Han et al. (as cited by Zhai et al.) demonstrated that TGF-β1 could induce DPSCs to differentiate into smooth muscle cells within 14 days. Another study showed that TGF-β1/β3 could differentiate DPSCs into chondrocytes (16).
Nerve growth factor (NGF), a member of the neurotrophin family, can signal neural or non-neural cells to differentiate. Catón et al. (as cited by Zhai et al.) showed that NGF is expressed in dental tissue and can lead to the proliferation and differentiation of dental stem cells, forming mineralized nodules in the odontogenesis process. Platelet-derived growth factor (PDGF) has also been shown to promote DPSC proliferation into odontoblasts and dentin-pulp complex regeneration (16).
Bone morphogenic proteins (BMPs), a subtype of the TGF-β family, play a significant role in tooth development and bone formation. Bone morphogenic proteins are involved in early odontogenesis and can influence the size, number, and shape of teeth.
Galler et al. (as cited by Diana et al.’s) study demonstrated that BMPs induce dental pulp stem cells and influence tooth morphology. In an in vitro study, it was discovered that certain subtypes of BMPs, such as BMP4 and BMP7, can affect dental pulp stem cell proliferation. For example, BMP7 can induce the formation of mineralized nodules, while BMP2 can promote dental pulp stem cells to differentiate into odontoblasts. Previous studies have shown that BMP2 not only promotes the proliferation and differentiation of dental pulp stem cells but also enhances dentinogenesis. As highlighted in our study, we introduced a novel, natural, and readily available signal for dentinogenesis, showing promising outcomes for future advancements in dental treatments. It was also demonstrated that obtaining dental pulp stem cells is relatively straightforward and can be achieved with the resources available today (17).
Regenerative dentistry is a modern field of research with significant potential, largely because dental pulp stem cells can be easily cultured and have the potential to differentiate into various dental tissues. These cells can be obtained from extracted teeth with minimal effort and cost. Growth factors and scaffolds can direct their proliferation and differentiation into other tissues. Dental pulp stem cells can regenerate into ectodermal, mesodermal, or dentin-pulp complexes when appropriately stimulated by growth factors and scaffolds, both in vitro and in vivo. If DPSCs are correctly induced by biological signals, it could lead to a revolution in regenerative research and their broader application in dentistry. Therefore, further research is essential, particularly in identifying the appropriate signals for each tissue conversion.
To achieve this, research must focus on identifying various combinations of signals or growth factors that yield efficient results in target tissues, thereby improving regeneration. This line of research in stem cell medicine requires further studies, especially in finding the suitable growth factors for dental tissue regeneration (17).
5.1. Conclusions
This study introduced a novel, natural, and readily available signal for dentinogenesis, demonstrating that DDS can be easily obtained in the lab and can be used as a stem cell signal for further studies. Significant progress has been made in novel dentistry approaches for tissue engineering and the regeneration of lost dental tissues. While several materials have been evaluated for their potential as growth and differentiation factors (signals) in previous research, further studies are needed to determine the most appropriate growth factor for each specific dental tissue.