1. Background
Acute pulmonary thromboembolism (PTE) is a potentially life-threatening condition that is often overlooked or misdiagnosed. It has been ranked as the third most prevalent cause of death from cardiovascular complaints, following myocardial infarction and cerebrovascular accident (1, 2). PTE is a leading preventable cause of death in hospitalized patients, with an estimated incidence of 100,000 to 180,000 deaths annually in the United States alone. According to the International Cooperative Pulmonary Embolism Registry (ICOPER), the three-month cumulative mortality rate associated with PTE can be as high as 17.4% (3). To improve patient outcomes and minimize unwarranted radiologic investigations, physicians should be properly trained to use validated PTE management algorithms. Clinical pre-test probability assessment is recommended to identify eligible patients. One of the most valid clinical decision rules is the Wells score criteria (Table 1). By applying the two-tiered model, patients with scores > 4 and scores ≤ 4 are categorized as “PTE-likely” and “PTE-unlikely” groups, respectively (4). In patients with low clinical probability, PTE can be excluded solely with negative D-dimer testing (5). However, patients with high clinical probability or those with high D-dimer levels should be further investigated by CTPA (6).
The emergence of CTPA in the late 1990s has significantly enhanced the evaluation of patients with suspected PTE. This technique is known as the choice of imaging modality with a sensitivity of 83% and a specificity of 96% for PTE detection (7, 8). However, earlier cohort studies estimated that approximately one-third of computed tomography scans are avoidable, and excessive use of CTPA over the past two decades has led to the overdiagnosis of PTE. While CTPA has minimally contributed to the reduction of PTE mortality rates, it has exposed patients to contrast-induced nephrotoxicity and carcinogenic exposure (9, 10).
2. Objectives
The current study aims to assess the rate of inappropriate CTPA use and physicians’ adherence to clinical prediction rules in the evaluation of suspected PTE at an academic tertiary center. The study aims to identify the extent of overuse of CTPA requests in this setting and explore potential factors contributing to this overuse.
3. Methods
3.1. Study Setting, Patients and Data Collection
We conducted a cross-sectional study in the Department of Pulmonary and Critical Care at the Shariati Hospital at Tehran University of Medical Sciences (TUMS) between April 2016 and March 2019. Our study was approved by the Institutional Review Board of TUMS. To ensure ethical research practices, we obtained written informed consent from all participants in our study. We collected medical records of 1058 patients aged 15 years and above who underwent CTPA for suspected acute PTE. Wells criteria were calculated retrospectively based on documented notes (Table 1). Then, the patients were categorized into those with high clinical probability (i.e., scores > 4) and low clinical probability (i.e., scores ≤ 4) of PTE. We examined age-adjusted D-dimer values to improve the accuracy of D-dimer testing in different age groups. The quantitative D-dimer testing was measured in nanograms per milliliter (ng/mL) using enzyme-linked immunoassay. In our study, we used a D-dimer cut-off value of greater than 255 ng/mL to determine a positive result. Additionally, CTPA results were classified as positive (definite diagnosis of acute PTE) and negative (absence of acute PTE) based on the consensus of two expert radiologists.
| Parameter | Point |
|---|---|
| Signs and symptoms of DVT | 3 |
| No alternative diagnosis better than PTE | 3 |
| Heart rate greater than 100 beats per minute | 1.5 |
| Immobilization for at least 3 days, or recent surgery in the previous 4 weeks | 1.5 |
| Prior history of DVT or PTE | 1.5 |
| Hemoptysis | 1 |
| Malignancy (on treatment within 6 months or palliative therapy) | 1 |
| Clinical probability of PTE | Score |
| Unlikely | ≤ 4 |
| Likely | > 4 |
Abbreviations; DVT, deep vein thrombosis; PTE, pulmonary thromboembolism.
3.2. Statistical Analysis
Table 2 presents descriptive statistics (mean ± (SD) of clinical and demographic data obtained from the participants of the study. The chi-square test was applied to compare the categorical variables (i.e., all variables in Table 2 except age) between the PTE-positive and PTE-negative groups, whereas an independent T-test was used to determine if the two groups were statistically different in age. Additionally, the odds ratio (OR) for clinical suspicion (based on Wells criteria) and PTE diagnosis with CTPA was reported. All statistical analyses were performed using SPSS version 26, and results were interpreted based on the alpha level of 0.05 as the statistical level of significance.
4. Results
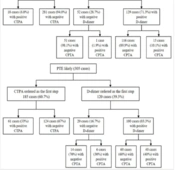
A total of 1058 patients suspected of acute PTE who underwent CTPA were initially selected for this study. Of these, 273 patients were excluded due to missing documentation and/or pregnancy (Figure 1). A total of 785 cases were retrospectively analyzed in this study. The average (SD) age of the participants was 53.9 ± (18.3) years with 54.3% being female. Patients’ characteristics, including demographics, symptoms, signs, and Wells criteria, are presented in Table 2.
| Variables | PTE-Positive (n = 139) | PTE-Negative (n = 646) | P-Value |
| Demographics | |||
| Age | 54.5 ± 18.5 | 53.7 ± 18.2 | 0.654 |
| Female | 67 (48.2) | 359 (55.6) | 0.133 |
| Symptoms, signs, wells criteria | |||
| Dyspnea | 121 (87.1) | 569 (88.1) | 0.774 |
| Tachycardia | 88 (63.3) | 325 (50.3) | 0.006 |
| Cough | 61 (43.9) | 281 (43.5) | > 0.99 |
| Chest pain | 70 (50.4) | 254 (39.3) | 0.018 |
| Fever | 22 (15.8) | 130 (20.1) | 0.287 |
| DVT signs & symptoms | 51 (36.7) | 51 (7.9) | < 0.001 |
| Hemoptysis | 23 (16.5) | 61 (9.4) | 0.022 |
| Syncope | 13 (9.4) | 32 (5) | 0.067 |
| Immobility for > 3 days or previous surgery within 4 weeks | 66 (47.5) | 247 (38.2) | 0.045 |
| Malignancy | 30 (21.6) | 164 (25.4) | 0.387 |
| Previous VTE | 33 (23.7) | 48 (7.4) | < 0.001 |
| Others | |||
| Positive D-dimer b result | 62 of 71 (87.3) | 190 of 262 (72.5) | 0.012 |
| Likely wells score | 107 (77.0) | 198 (30.7) | < 0.001 |
Abbreviations; DVT, deep vein thrombosis; VTE, venous thromboembolism.
a Values are expressed as mean ± SD or No. (%).
b Data of D-dimer were available for 333 patients (71 patients in PTE-positive group and 262 in PTE-negative group).
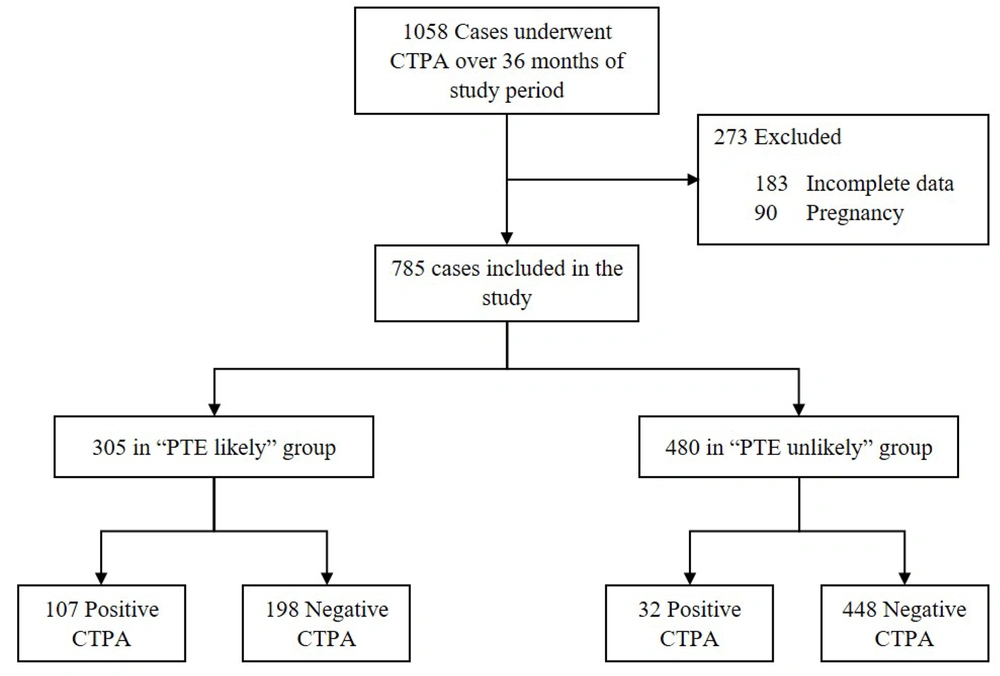
Wells scores were calculated for all patients. Individuals with PTE-positive results had significantly higher Wells scores compared to those with PTE-negative results (P < 0.001). Patients were categorized into the “PTE-unlikely” (n = 480) and the “PTE-likely” (n = 305) groups based on the Wells scores (Figure 2). Out of the total 785 patients, 139 (17.7 %) were diagnosed with acute PTE on CTPA. The prevalence of PTE was significantly higher in the “PTE-likely” group compared to the “PTE-unlikely” group, with an odds ratio of 7.6 (95% CI = [4.92 - 11.61], P < 0.001).
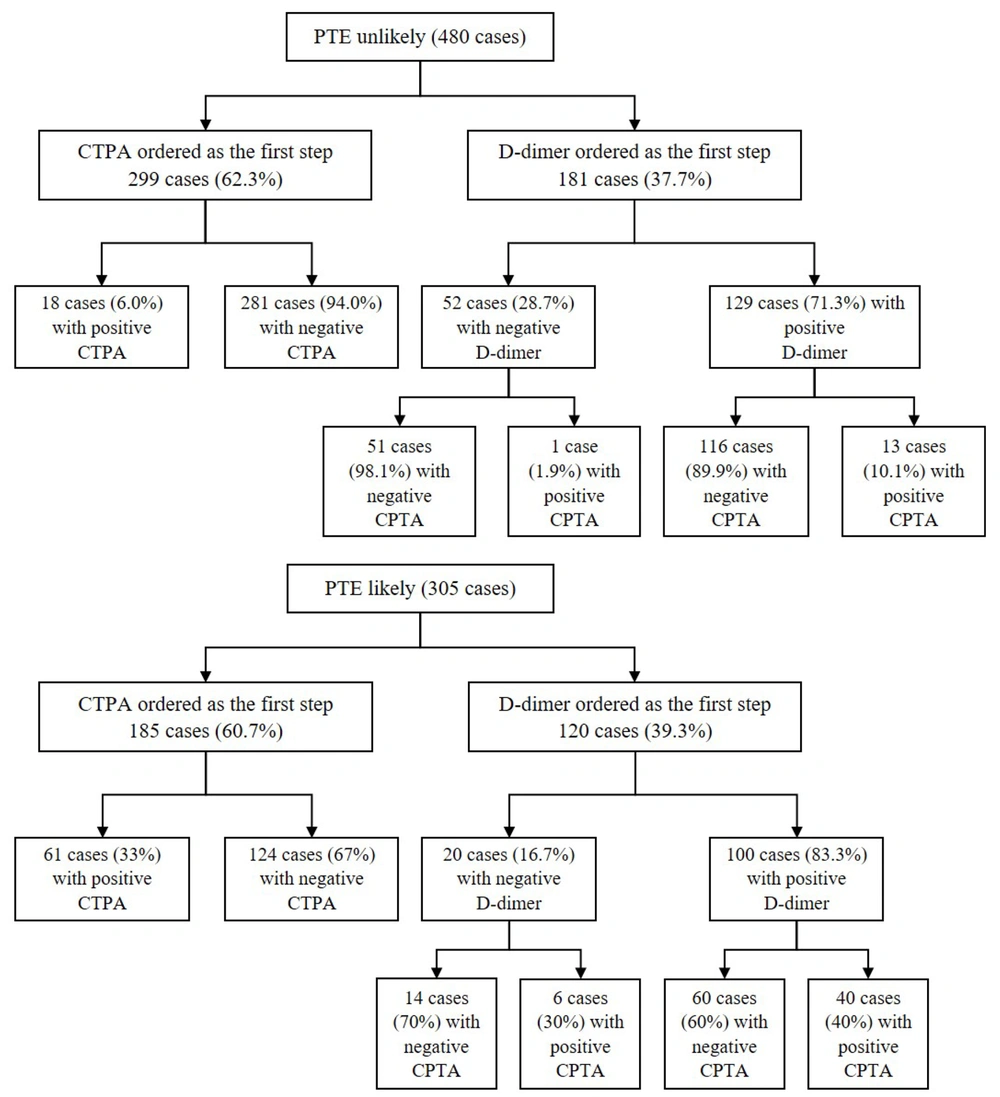
In the "PTE-unlikely" group, 299 (62.3 %) patients underwent CTPA directly despite recommendations to order a D-dimer test first, of whom 281 (94 %) cases had negative results for acute PTE (Figure 2). On the other hand, in the same group, 181 (37.7 %) cases underwent a D-dimer assay appropriately as the first step of diagnosis. Among these cases, 52 (28.7 %) had negative D-dimer values, and only one patient was followed by a positive CTPA report (Figure 2). In the "PTE-likely" group, 107 (35.1 %) patients showed positive results for acute PTE on CTPA (Figure 1). However, 120 (39.3 %) cases underwent D-dimer assays as the first step of diagnosis, which is not recommended for patients with a high clinical pretest probability (Figure 2).
Overall, the study found that out of a total of 785 CTPA requests, 351 (44.7 %) were found to be avoidable. Specifically, 299 of the CTPA requests were made for "PTE-unlikely" patients as the first step of diagnosis, while 52 of the CTPA requests were made for "PTE-unlikely" patients with negative D-dimer results.
5. Discussion
The present study highlights the high rate of avoidable overuse of CTPA in patients with suspected acute PTE, with 44.7% of patients undergoing CTPA unnecessarily. This overutilization in our medical center may be attributed to several factors, including a lack of physicians’ knowledge, fear of misdiagnosis, and ease of access to CTPA procedures. In the current study, out of 785 patients, 139 (17.7 %) were diagnosed with acute PTE. The diagnostic yield of CTPA has been reported to vary worldwide, with investigations conducted in the United States showing yields in the range of 6 to 10 % (11-16). Our study reported a higher positivity rate compared to findings in prior literature in the United States and other published reports on Asian populations (17-19).
The study also found a substantial rate of non-adherence in the current diagnostic algorithms, particularly regarding the utilization of D-dimer assay before proceeding to CTPA in approaching patients with low clinical pre-test probability. In our study, D-dimer was not ordered before CTPA request in the majority of “PTE-unlikely” patients (62.3 %). This finding is consistent with earlier literature, which has also reported similar patterns of non-adherence to diagnostic algorithms (14, 20, 21). As validated in earlier research, D-dimer has a negative predictive value of up to 100% for the “PTE-unlikely” patients; thereby, PTE can be safely excluded without resorting to further imaging studies (22-25). Our findings are consistent with these literatures as we observed a low rate of positive CTPA results (1.9 %) in “PTE-unlikely” individuals despite the negative D-dimer. The rates of CTPA requests in “PTE-unlikely" patients with negative D-dimer results in our study are also comparable to the outcomes of prior research, which ranged between 3 and 13.2 % (14, 16, 20, 26).
The potential disadvantages of CTPA, including radiation exposure and contrast-induced nephrotoxicity, emphasize the importance of appropriate utilization of this imaging modality. A prospective cohort study found that a significant proportion of patients who underwent CTPA for PTE assessment developed contrast-induced nephropathy. Additionally, the overuse of CTPA burdens the healthcare system (27-29). Therefore, although prompt PTE diagnosis is required, unnecessary CTPA requests should be prevented.
Our findings emphasize the need for further education and training of physicians to improve adherence to evidence-based guidelines and protocols. Supporting evidence for this could be found in a previous retrospective study on PTE-suspected Turkish patients, which demonstrated that the use of CTPA declined by up to 26.6 % through the proper use of diagnostic protocols (30).
5.1. Study Limitations
Several limitations need to be addressed in this study. Firstly, despite implementing a large sample size, the exclusion of 183 patients (17.2 %) due to incomplete data was suboptimal. Secondly, the assessment of PTE probability was conducted retrospectively by researchers rather than concurrently during the patient’s screening. This may have introduced bias into the study and affected the accuracy of the results. Future studies should aim to address this limitation by conducting prospective studies that assess PTE probability concurrently with the patient's screening. Lastly, patients with negative CTPA reports were not followed up to determine whether factors other than PTE may have caused negative CTPA results. Future studies should aim to address these limitations by conducting follow-up studies to provide more robust evidence on the appropriate use of CTPA for PTE diagnosis.
5.2. Conclusions
In conclusion, our study highlights the need for improved adherence to evidence-based guidelines in the evaluation of patients with suspected acute PTE. By promoting the appropriate use of validated diagnostic algorithms and enhancing clinical decision-making, we can prevent unnecessary CTPA requests and minimize avoidable overuse of imaging.