1. Background
The oral cavity, as the first part of the digestive system, is the most complex and accessible ecosystem of the human body. Human oral flora includes more than 700 microorganisms, and more than 50% of them are uncultivable (1). Various infections caused by obligate anaerobic microorganisms, along with facultative anaerobes, especially gram-positive streptococcal bacteria in the oral cavity called Streptococcus viridans, are known as the main pathogen responsible for dentoalveolar infections, pericoronitis, and periodontitis (2). Mechanical treatments are effective in reducing the aggregation of local infections but cannot reach all infected areas. Therefore, antibiotic treatment is considered an integral part of treatment in oral cavity infections (3-6).
Penicillin is considered the first line of treatment for odontogenic infections. If the patient does not respond to penicillin during the early stages of odontogenic infection, there is a high probability of bacterial resistance. Bacterial resistance to penicillin is often due to the production of beta-lactamase by bacteria. In cases of penicillin allergy, beta-lactamase-resistant antibiotics should be prescribed to the patient. These drugs include clindamycin or amoxicillin with clavulanic acid (co-amoxiclav) (7). Clindamycin has excellent broad-spectrum activity. Its effectiveness in the treatment of odontogenic infections is comparable to penicillin (7). This drug has been successfully used to treat infections that have failed to respond to other drugs (8). The broad-spectrum coverage of clindamycin, with its excellent clinical effect, has made this drug the drug of choice in the treatment of odontogenic infections as an alternative to penicillin V in some standard guidelines (7).
In the antibiotic prophylaxis protocol published in 2007, the American Heart Association suggested the use of clindamycin over erythromycin in penicillin-sensitive individuals in cases of infective endocarditis prophylaxis (9). However, in the new antibiotic prophylaxis protocol published by this association in 2021, the use of cephalexin, azithromycin, clarithromycin, and doxycycline instead of clindamycin is suggested before invasive dental treatments in cases of sensitivity to penicillin. Therefore, it is important to analyze this replacement after 14 years of subjection from the previous prophylaxis protocol (10). The resistance of a pathogenic bacterium to antibiotics is not the same in different communities (11). Most of these studies have been conducted in developed countries; however, the behaviors leading to the emergence of antibiotic-resistant bacteria are more common in third-world countries (12).
2. Objectives
By investigating the resistance of streptococci samples isolated from the root surface of extracted teeth against two more common antibiotics prescribed for infection treatments of dental origin in the Iranian community, this study aimed to help prescribe a more appropriate and precise antibiotic choice for the treatment of oral infections.
3. Methods
This descriptive, analytical, and cross-sectional study was conducted in vitro in 2021. The samples were taken from individuals who attended tooth extraction in the Oral and Maxillofacial Department of Yazd Dental School, Yazd Shahid Sadoughi University of Medical Sciences, Yazd, Iran. All methods were conducted in accordance with the ethical standards of the Declaration of Helsinki. Considering the confidence level of 95% and the test power of 80% and according to previous similar studies (13, 14) and the significant difference in the report of penicillin resistance (11.1%) and clindamycin resistance (33.3%) (15), 45 samples were needed to assess the minimum antibiotic sensitivity of bacteria to clindamycin and penicillin.
3.1. Inclusion and Exclusion Criteria
No subject had received antibiotics, steroids, or immune therapy or used any antiseptic mouthwashes for one week before the sampling procedure. Having a history of any systemic disease or malignancy precluded entry into the study. If there was evidence of chronic infection or periapical abscess during clinical and radiographic evaluations, the sample was not included in the study. If the sample was contaminated during the extraction process, transfer process, and cultivation process, it was excluded from the study. Out of the 50 samples taken, 46 met the inclusion and exclusion criteria, and 4 were excluded.
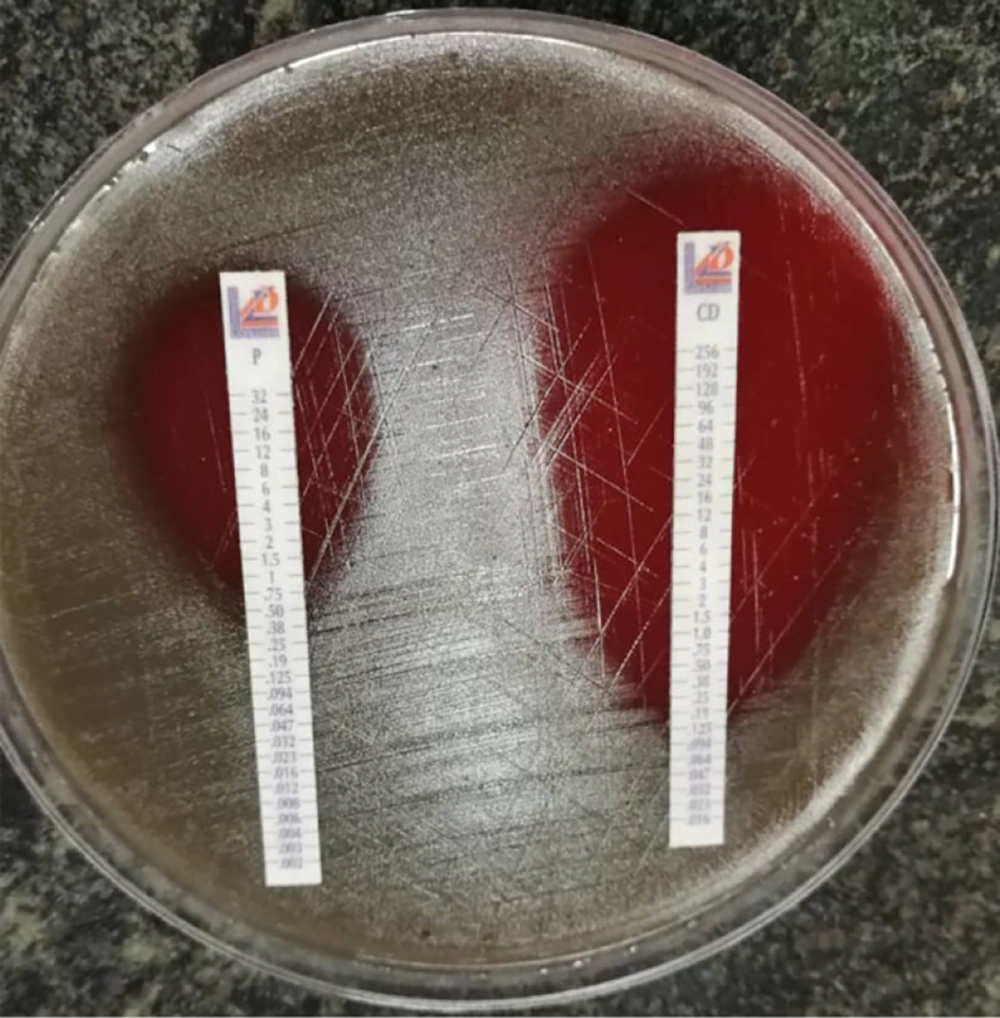
The swab was gently pulled over the surface of the root as far as possible away from the bloody area. The swabs were placed in a tube containing tryptic soy broth (TSB) medium (Merck, Germany) and transported to the microbiology laboratory within one hour at most. The samples were cultured in blood agar medium (Merck, Germany) containing 5% sheep blood in isolation, and after placing the plates in a CO2 candle jar, they were incubated at 35 - 37ºC for 48 hours. By using gram staining (Bahar Afshan, Iran), checking the presence of hemolysis around the colonies, and biochemical tests, such as catalase, the identity was determined to the extent of the Streptococcus genus. For the determination of minimum inhibitory concentration (MIC), the E-test (Liofilchem, Italy) antibiotic sensitivity method was used based on the Clinical and Laboratory Standards Institute (CLSI) 2021 protocols (Table 1).
| Anti-microbial Agent | S | I | R |
|---|---|---|---|
| Penicillin | ≤ 0.12 | 0.25 ≤ MIC ≤ 2 | ≥ 4 |
| Clindamycin | ≤ 0.25 | 0.5 | ≥ 1 |
Minimum Inhibitory Concentration Breakpoints, μg/mL
In the next step, a bacterial suspension tube with a concentration equivalent to half McFarland (1.5 × 108 bacteria/mL) was prepared from a fresh culture (24 hours) in a tube containing sterile physiological serum and placed on the plates containing the Mueller Hinton blood agar (Merck, Germany) medium by lawn culturing method.
Then, E-test strips of antibiotics, penicillin V and clindamycin (Liofilchem, Italy), were placed separately on the culture medium. After incubating the plates at 35°C for 18 - 24 hours, the intersection of the lack of bacterial growth aura was determined, and the antibiotic concentration at the intersection point was considered as MIC by micrograms per milliliter. (Figures 1 and 2). The MICs obtained for the two antibiotics, penicillin and clindamycin, were compared to the numbers in the CLSI 2021 protocol table (Table 1).
3.2. Statistical Analysis
After collecting the data, they were entered into SPSS software (version 23). The required tables and indexes were prepared, and non-parametric Wilcoxon tests were used for statistical comparison of the data due to non-compliance of data from normal distribution.
4. Results
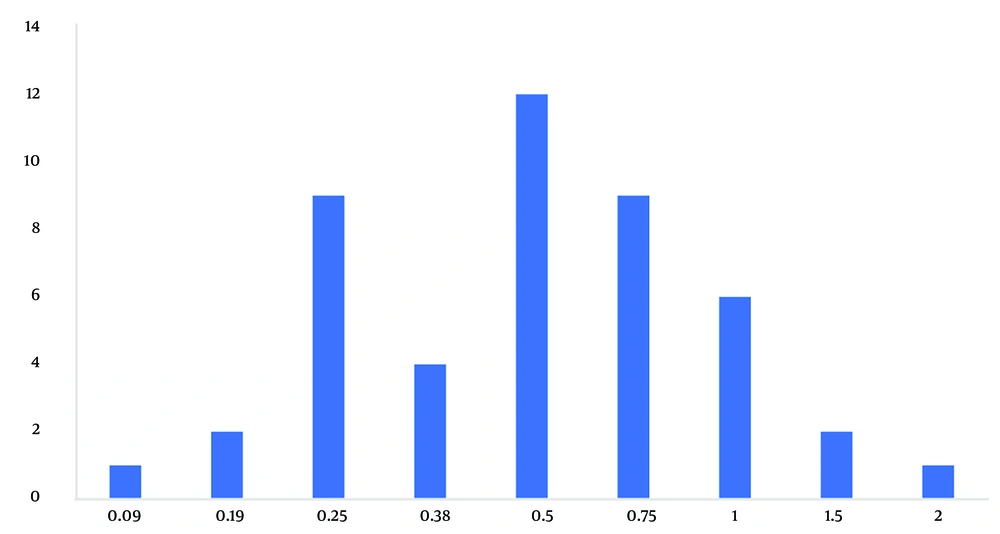
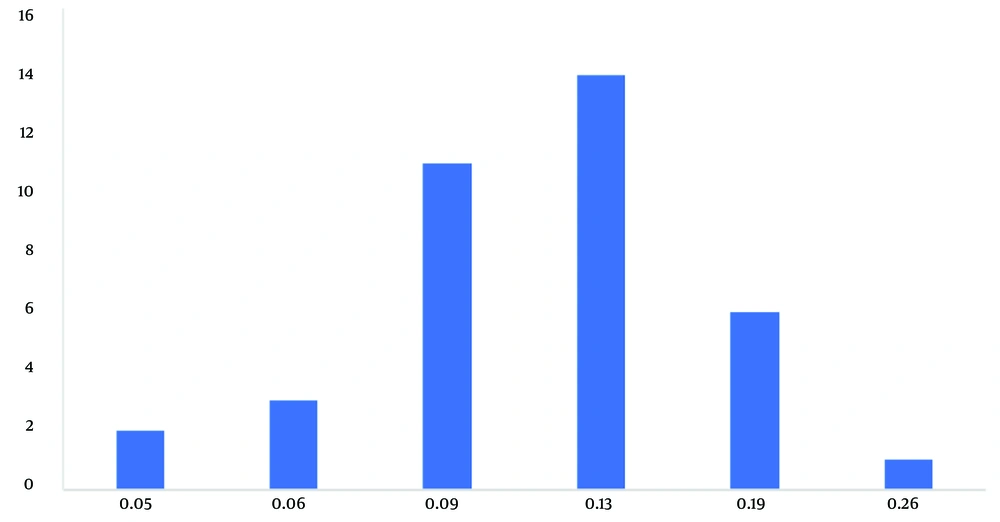
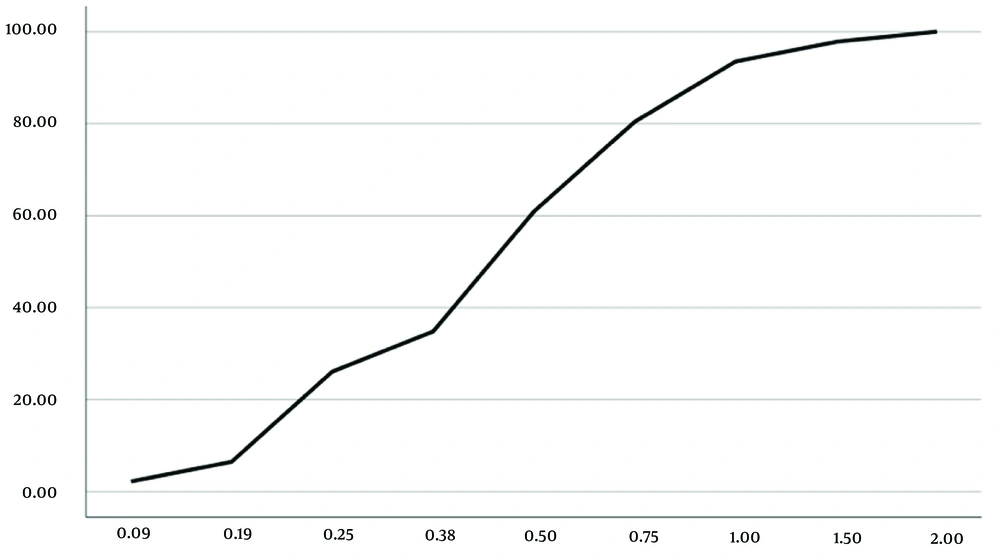
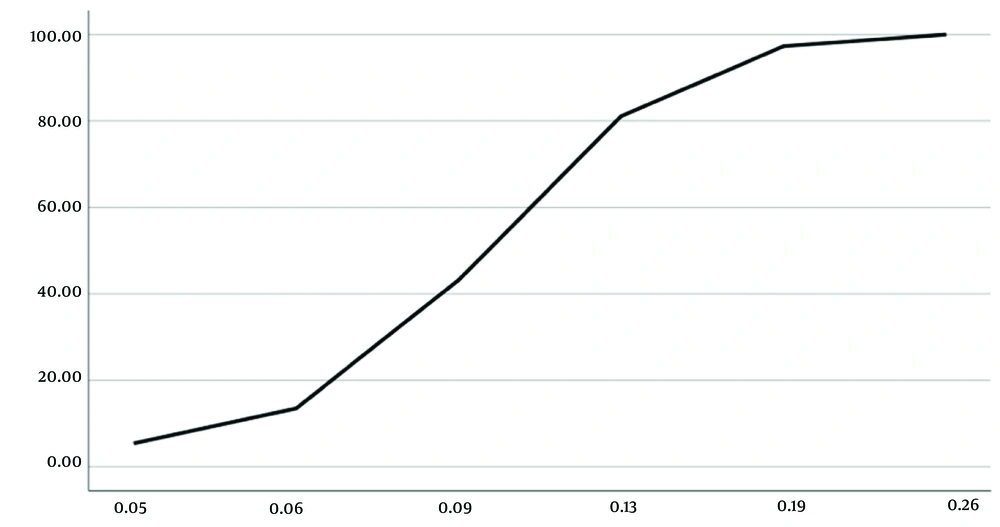
The frequency distributions of the sensitivity and resistance (Figures 3 and 4) and cumulative distributions of the sensitivity and resistance of the two studied antibiotics are shown below (Figures 5 and 6). Finally, comparative study results are shown below (Tables 2 , 3, and 4).
| Antibiotic | MIC Domain | MIC 50 | MIC 90 | Interquartile Domain |
|---|---|---|---|---|
| Penicillin | 1.91 | 0.5 | 1.0 | 0.5 |
| Clindamycin | 0.21 | 0.125 | 0.19 | 0.031 |
The Range, MIC 50, MIC 90, and the Interquartile Range of the Two Studied Antibiotics
| Antibiotic | Interquartile Range |
|---|---|
| Penicillin | 0.5 (0.25 - 0.75) |
| Clindamycin | 0.125 (0.094 - 0.125) |
The Interquartile Range of the Minimum Inhibitory Concentration (MIC) of the Two Studied Antibiotics
P-value = 0.0001 Wilcoxon test.
| Antibiotic | Sensitive | Intermediate | Resistant |
|---|---|---|---|
| Penicillin | 2 (4.4) | 44 (95.6) | 0 (0) |
| Clindamycin | 36 (78.2) | 0 (0) | 10 (21.8) |
5. Discussion
Antibiotic resistance has become a global public health crisis. Dentists are aware of the phenomenon of antibiotic resistance and play an important role in preventing the emergence of antibiotic resistance (16). In the present study, the samples taken from the root surface extracted teeth were cultured for analyzing streptococci spp., which is due to the presence of this group of bacteria in the normal flora of the oral cavity, oral infections, periodontitis, and pericoronitis. The range of MIC of streptococcal spp. isolated from the root surface in the current study for penicillin was between 0.09 and 2 μg/mL, which, compared to older studies (3, 17-23), has increased, which indicates an increased ability of this type of bacteria to resist, compared to the past. Moreover, the maximum MIC level in the present study is still in the range that can be treated with this drug, and it seems that in cases where the immune system of individuals is competent, it can still be used as a first-line drug for the treatment of oral infections.
By training and increasing the skills of doctors in relation to the prescription of antibiotic agents and improving the knowledge of patients to use antibiotic drugs appropriately, it is possible to prevent the development of more resistance to this antibiotic and use penicillin for the treatment of oral infections, and it is still promising. The standard deviation observed in MIC for penicillin was 0.6, which is more than what was observed for clindamycin (0.12). The higher deviation of MIC against penicillin shows that despite the much longer duration of extraction and use of this antibiotic, there are still many sensitive cases with multiple MICs. According to Figure 3, it can be concluded that the current average level of resistance to penicillin is more widespread, and the largest number of samples were inhibited at MIC equal to 0.5 μg/mL. Moreover, it is possible to increase the dose more for penicillin to inhibit more of these isolates; however, this dose adjustment is not possible for clindamycin because side effects and toxicity, along with increasing the dose, reduce the therapeutic window of this antibiotic.
The range of MIC of streptococci spp. isolated from the root surface in the present study for clindamycin was between 0.06 and 256 μg/mL, which has increased, compared to other studies (3, 17-23), which indicates an increase in resistance of this type of bacteria, compared to the past. In the present study, the maximum MIC did not inhibit some of the isolated streptococci spp., and it seems that in cases where there is resistance to this drug (21.8%), it is not effective either as a therapeutic antibiotic or as a prophylactic antibiotic. However, in cases where there was sensitivity to this drug, it was effective with a significantly lower MIC than penicillin. Therefore, in sensitive cases, this drug can reduce the duration of hospitalization and reduce related treatment costs.
The MIC 50 and MIC 90 of streptococcal spp. isolated from the root surface in the present study was reported to be 0.5 μg/mL and 1 μg/mL, respectively, for penicillin and 0.125 μg/mL and 0.19 μg/mL, respectively, for clindamycin, which is much higher than similar studies (2, 3, 17, 19, 23) in developing countries. The diversity observed in the MICs reported in studies of aerobic streptococcal isolates for clindamycin can be considered to be related to the greater spectrum of activity of this antibiotic on obligate anaerobic microorganisms. However, this drug is among the alternatives to antibiotic prophylaxis, and further studies are needed in relation to assessing the effectiveness of this antibiotic in the common types of streptococci spp. that cause infective endocarditis and cases of prosthetic joint infection.
Most of the studies reported the resistance of aerobic isolates to penicillin within the range of 10 - 60% (2, 13-15, 24-32). In the present study, only one sample was sensitive to penicillin, and the other samples (95.6%) did not show resistance to penicillin; however, they were in the MIC range of intermediate sensitivity. The range of resistance of facultative aerobic and anaerobic isolates to clindamycin has been reported between 0 - 65% in the reviewed articles. In the present study, 21.8% of the examined samples showed resistance to clindamycin. It can be concluded that in some cases, aerobic isolates were resistant to clindamycin; however, there was intermediate sensitivity to penicillin, which can lead to the conclusion that the effect of penicillin on aerobic types of polymicrobial infections of the oral cavity is more than clindamycin. In 78.2% of the samples, the sensitivity of the same type of bacteria to clindamycin was observed, and the same samples were intermediately sensitive to penicillin. Therefore, the elimination of clindamycin in the new antibiotic prophylaxis protocol published in 2021 by the American Heart Association makes sense.
5.1. Conclusions
According to the present study, penicillin can still be used as a first-line drug to prevent infections in the mouth, jaw, and face. Today, this drug has been forgotten for the treatment of oral infections in Iran, although penicillin is still the first line of treatment in most antibiotic treatment guidelines for oral infections. It seems that except in limited cases where there is resistance to clindamycin, this antibiotic is a more effective drug to control the bacteria in the mouth. However, in some cases, aerobic isolates showed intermediate sensitivity to penicillin V; instead, they were resistant to clindamycin.